Abstract
Studies on the pathogenesis of Salmonella enterica serovar Typhimurium infections in mice have revealed the presence of two prominent virulence characteristics—the invasion of the nonphagocytic cells to penetrate the intestinal epithelium and the proliferation within host phagocytic cells to cause a systemic spread and the colonization of host organs. We have recently demonstrated that the ATP-dependent Lon protease of S. enterica serovar Typhimurium negatively regulates the efficiency of invasion of epithelial cells and the expression of invasion genes (A. Takaya et al., J. Bacteriol. 184:224-232, 2002). This study was performed to reveal the contribution of the Lon protease to the virulence of S. enterica serovar Typhimurium in mice. Determination of 50% lethal doses for the lon disruption mutant and wild-type strain revealed that the mutant was highly attenuated when administered either orally or intraperitoneally to BALB/c mice. The mutant was also found to be able to reach extraintestinal sites but unable to proliferate efficiently within the spleen and cause lethal systemic disease of mice. Macrophage survival assays revealed that the lon disruption mutant could not survive or proliferate within murine macrophages. In addition, the mutant showed extremely increased susceptibility to hydrogen peroxide, which contributes to the bactericidal capacity of phagocytes. The mutant also showed increased sensitivity to acidic conditions. Taken together, the impaired ability of the lon disruption mutant to survive and grow in macrophages could be due to the enhanced susceptibility to the oxygen-dependent killing mechanism associated with respiratory burst and the low phagosomal pH. These results suggest that the Lon protease is essentially involved in the systemic infection of mice with S. enterica serovar Typhimurium, which can be fatal. Of further interest is the finding that the lon disruption mutant persists in the BALB/c mice for long periods without causing an overwhelming systemic infection.
Salmonella enterica causes a spectrum of diseases ranging from mild gastroenteritis (serovar Typhimurium and serovar Enteritidis) to a severe systemic infection which can be fatal (serovar Typhi and serovar Paratyphi). A useful tool for investigation of the systemic form of salmonellosis is the murine model of typhoid-like disease caused by S. enterica serovar Typhimurium. Following oral infection of mice, bacteria adhere to and invade cells of the intestinal epithelium, survive in blood, proliferate in macrophages, and access systemic sites through the lymphatic and blood circulation systems (4, 15). Numerous genes that contribute to S. enterica serovar Typhimurium virulence have been identified and determined to be clustered together on Salmonella pathogenicity islands (SPI) (17, 19). SPI1, located at centisome 63 on the Salmonella chromosome, and SPI2, located at centisome 30, are major SPI that encode structurally similar but functionally distinct type III secretion systems which translocate the effector proteins directly into host cells to contribute to pathogenesis (23). The function of SPI1 is required for the initial stage of salmonellosis, that is, the invasion of the nonphagocytic cells and penetration of the gastrointestinal epithelium. The SPI2 function is involved in the latter stages of the infection, that is, survival and proliferation in host phagocytic cells to cause a systemic spread and the colonization of host organs. The expression of SPI1 and SPI2 genes appears to be regulated at several stages in a complex manner by various regulators within and outside SPI (22, 30). We have recently reported that the efficiency of invasion of epithelial cells and the expression of invasion genes carried on the SPI1 are negatively regulated by the ATP-dependent Lon protease (43). Lon is a member of four families of ATP-dependent proteases—including the Clp family (ClpAP and ClpXP), HslVU, and FtsH—that have been well characterized in bacteria (7, 16, 40). These are also known as stress proteins, which are induced in response to unfavorable conditions such as heat shock and other stressful situations (33). In Escherichia coli, Lon is thought to be a major contributor to the elimination of stress-damaged proteins (29). In addition to its function in housekeeping, Lon has been shown to perform important regulatory functions in bacterial cells by controlling the availability of critical regulatory proteins that control gene expression. For instance, Lon is involved in the regulation of the SOS response in E. coli through its capacity to degrade SulA, an inhibitor of cell division, thereby allowing cells to resume division after the physiological response to DNA damage (39). Furthermore, Lon participates in the production of capsular polysaccharide by degrading RcsA, which is a transcriptional activator of the biosynthetic genes of capsular polysaccharide (45).
SPI1 is a contiguous 40-kb region of the chromosome of S. enterica serovar Typhimurium, and 39 genes have been identified within the SPI1 at present (19, 32). A central regulator of SPI1 gene expression is HilA, a member of the OmpR/ToxR family of transcriptional activators (1). HilA directly activates promoters of SPI1 genes encoding the type III apparatus and secreted effectors and the transcriptional regulator InvF, which also activates the transcription of effector genes (31). Therefore, the sequential action of the SPI1-encoded regulatory factors, HilA and InvF, is thought to coordinate the expression of many genes required for Salmonella invasion. In our previous study, the lon mutant derived from S. enterica serovar Typhimurium χ3306 showed a dramatic enhancement in the transcription of SPI1-regulator genes, hilA and invF, and effector genes, sipA and sipC, suggesting that the lon probably regulates SPI1 gene expression by proteolysis of putative factors required for activation of hilA expression (43).
In the present study, to know whether the dramatic increase in the ability to invade epithelial cells conferred by the lon mutation affects the pathogenesis of S. enterica serovar Typhimurium, we characterized both in vitro and in vivo phenotypes of the mutant, including the degree of virulence in mice inoculated either orally, subcutaneously, or intraperitoneally (i.p.). Consequently, we revealed that, in contrast to its highly effective invasiveness, the lon mutant is unable to establish a lethal systemic infection in mice.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth condition.
The S. enterica serovar Typhimurium virulent strains used in this study were χ3306 (nalidixic acid resistant), which was derived from strain SR11 (18). The following derivatives from the strain χ3306 were also used: CS2022 (lon::Cm mutant of χ3306), CS2099 (CS2022 harboring pTKY530), CS2239 (CS2022 harboring pMW119), and CS2347 (χ3306 harboring pMW119). The plasmid pTKY530 contains an entire copy of the χ3306 lon gene which was previously cloned on a low-copy-number plasmid pMW119. Bacterial derivatives from χ3306 and plasmid pTKY530 were previously constructed (43). Bacteria were grown at 37°C in L-broth (1% Bacto Tryptone [Difco, Detroit, Mich.], 0.5% Bacto Yeast extract [Difco], 0.5% sodium chloride [pH 7.4]) with shaking or on L-agar. The media were supplemented with chloramphenicol (25 μg/ml),), ampicillin (25 μg/ml), and/or nalidixic acid (25 μg/ml), when necessary.
Assay for mouse virulence.
S. enterica serovar Typhimurium cells grown in L-broth at 37°C to late exponential growth phase were centrifuged at 8,000 × g for 10 min at room temperature and suspended in phosphate-buffered saline, pH 7.0, containing 0.01% (wt/vol) gelatin (BSG). The actual number of bacteria present was determined by viable-cell counting. Seven-week-old female BALB/c mice (Charles River Japan, Yokohama, Japan) were orally or subcutaneously inoculated. At indicated points after inoculation, the spleens, mesenteric lymph nodes, or Peyer's patches were aseptically removed and then homogenized in BSG. The number of viable bacteria in the organs of infected mice was determined by plating serial 10-fold dilutions of the homogenates on L-agar plates. Colonies were routinely counted 18 to 24 h later. Seven-week-old female BALB/c mice were also used to determine the oral and i.p. 50% lethal doses (LD50s) of serovar Typhimurium χ3306 and CS2022 (lon::Cmr mutant). The LD50 was calculated by the method of Reed and Muench (37).
Assay for survival and growth in macrophages.
RAW264.7 macrophage cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Grand Island, N.Y.) containing 10% fetal calf serum (FCS) and 4 mM l-glutamine at 37°C. A total of 4 × 105 cells in each well of 24-well plates were challenged with S. enterica serovar Typhimurium strains at a multiplicity of infection of 1. The plates were centrifuged for 5 min at 500 × g to enhance and synchronize infection. The cells were incubated for 30 min at 37°C to permit phagocytosis, and the free bacteria were removed by three washes with Hanks buffered salt solution (HBSS). DMEM containing 10% FCS and 100 μg of gentamicin per ml was added, and the cells were incubated for 1.5 h at 37°C. The cells were washed with the prewarmed HBSS three times, and this was followed by incubation with DMEM containing 10% FCS and 10 μg of gentamicin per ml at 37°C. Wells were sampled at various times after inoculation by aspirating the medium, performing three washings with HBSS, and lysing the contents of each well with PBS containing 0.2% Triton X-100. The triplicate samples were plated individually after appropriate dilutions.
Assay for sensitivity of S. enterica serovar Typhimurium to hydrogen peroxide and acidic conditions.
For assaying the sensitivity to hydrogen peroxide, exponentially growing cells were exposed to 15 mM hydrogen peroxide in L-broth and then incubated at 37°C. After serial dilution at the indicated times, samples were transferred to L-agar plates to determine the number of viable cells. To examine the survival under acidic conditions, exponentially growing cells in L-broth (pH 7.4) were diluted 1:100 into L-broth at pH 4.0 or 3.5, which was previously adjusted with citric acid, and then incubated 37°C. After 30 min, bacterial cells were diluted into L-broth (pH 7.4) and then transferred to the L-agar plate to determine the number of viable cells.
RESULTS
Assessment of virulence of S. enterica serovar Typhimurium lon disruption mutant by infection of BALB/c mice.
One assessment of the virulence of S. enterica serovar Typhimurium is the ability of the bacterium to establish a lethal systemic infection in mice. To test whether the lon locus is required for the systemic infection of S. enterica serovar Typhimurium in mice, the oral and i.p. LD50s of serovar Typhimurium virulent strain χ3306 (lon+) and the lon disruption derivative, CS2022 (lon::Cmr), were estimated (Table 1). Consistent with previous findings (18), the virulent strain was found to have an i.p. LD50 of fewer than 50 organisms for BALB/c mice. In contrast, the i.p. LD50 of CS2022 was found to be ∼100,000-fold higher than that of χ3306. Values of oral LD50 also showed that CS2022 was highly attenuated.
TABLE 1.
Mouse LD50s for wild type and lon disruption mutanta
| Strain | Relevant genotype of strain | LD50 (CFU)
|
|
|---|---|---|---|
| oral | i.p. | ||
| χ3306 | lon+ | 3 × 105 | <50 |
| CS2022 | lon:Cmr | >5.0 × 108 | 2.4 × 106 |
Determination for BALB/c mice by the methods of Reed and Muench (37).
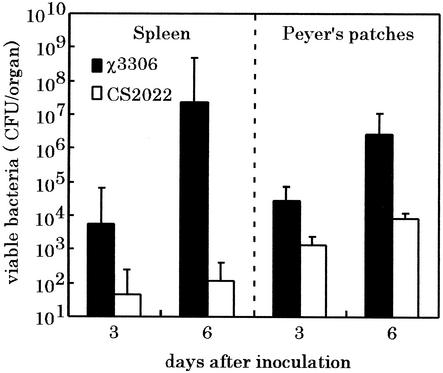
To determine whether the loss of virulence was due to an ability of the serovar Typhimurium lon disruption strain to colonize the spleen of mice, the numbers of viable bacteria in the spleen of groups of mice infected with either the parent or mutant strain were assessed. BALB/c mice were inoculated orally with 5 × 108 CFU of strain χ3306 or CS2022. The number of bacteria in spleens was assessed on days 3 and 6 after inoculation (Fig. 1). The wild-type strain χ3306 colonized the spleen in large numbers, resulting in more than 107 bacteria at 6 days after inoculation. In contrast, the strain CS2022 was present in the spleen in much lower numbers than the strain χ3306. We have previously demonstrated that the disruption of lon does not impair the ability of serovar Typhimurium to invade Intestine-407 cultured cells in vitro (43). To test whether the lon mutant can penetrate the intestinal epithelium in mice, the number of viable bacteria in the Peyer's patches was also counted at day 3 and 6 after inoculation. The organisms of strain CS2022 were detected in the Peyer's patches efficiently at levels of ∼100-fold higher than those in the spleens. These results suggest that the lon disruption mutant was able to penetrate the intestinal epithelium and reach extraintestinal sites.
FIG. 1.
Numbers of viable bacteria in the tissues of BALB/c mice after infection with wild-type strain and lon disruption strain of S. enterica serovar Typhimurium. A group of five mice was challenged orally with 5 × 108 CFU of strains χ3306 (lon+) or CS2022 (lon:: Cmr), and the numbers of bacteria recovered from the Peyer's patches and spleens were determined on days 3 and 6 after administration. The error bars indicate the standard deviation of the means of these counts.
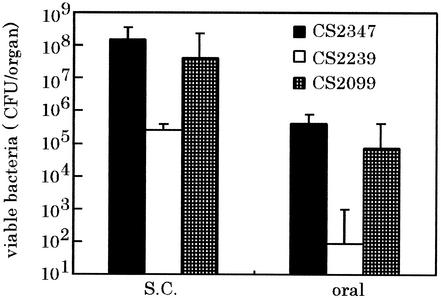
To confirm that the diminished ability to cause a systemic infection of the strain CS2022 is due to the lon disruption, a functional lon was provided in trans by introduction of a low-copy-number plasmid carrying lon gene of χ3306 and tested for complementation of lon disruption by assessing the ability to colonize the spleen of BALB/c mice. The numbers of bacteria recovered from the spleen on day 6 after inoculation are shown in Fig. 2. Consistent with the previous results shown in Fig. 1, mice orally infected with strain CS2347 (lon+/pMW119) and CS2239 (lon::Cmr/pMW119) had approximately 106 bacteria and 102 bacteria in the spleen, respectively. The diminished ability of CS2022 to cause the systemic infection was fully restored by providing a functional copy of lon as determined by infection with strain CS2099 (lon::Cmr/pTKY530). Since the lon-disruption results in the enhanced invasion of Salmonella into Intestine-407 cultured cells (43), the virulence of these strains was also estimated following the subcutaneous administration of BALB/c mice to avoid the step of invasion of bacteria into epithelial cells. The number of bacteria in the spleen was assessed on day 4 after challenge (Fig. 2). Again, the lon disruption mutant exhibited an impaired ability to cause lethal systemic decrease in mice inoculated by subcutaneous route, and the inability to express virulence was fully restored by providing a functional copy. From these results, it is concluded that the Lon protease is essentially required for the systemic Salmonella infection of mice.
FIG. 2.
Effect of suppression of lon disruption on colonization of the spleens in mice by providing a functional lon in trans. A group of five mice was challenged orally (oral) or subcutaneously (S.C.) with S. enterica serovar Typhimurium strain CS2347 (lon+/pMW119+), CS2239, (lon:: Cmr/pMW119+), or CS2099 (lon:: Cmr/pTKY530+). On day 4 after subcutaneous inoculation with 2 × 105 of organisms or on day 6 after oral inoculation with 5 × 108 organisms, the numbers of bacteria recovered from spleens were determined. The error bars indicate the standard deviation of the means of these numbers.
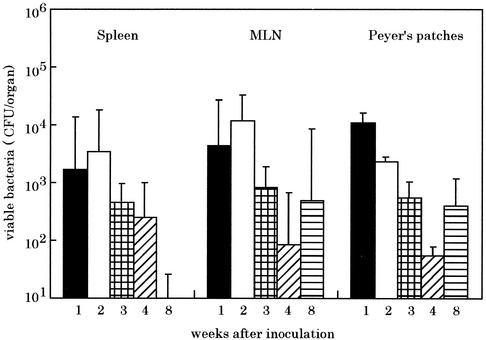
Unlike mice infected with the wild-type strain, which died, all mice challenged with the lon disruption mutant appeared to be much more capable of controlling infection and survived beyond day 6 after infection. In order to understand further the reduced virulence of strain CS2022, the ability of bacteria to colonize, survive, and replicate in different murine tissues was monitored for up to 8 weeks after oral inoculation (Fig. 3). The mice surviving infection with the strain CS2022 could not completely clear the infection by at least 8 weeks after initial inoculation. These results demonstrate that the S. enterica serovar Typhimurium lon disruption mutant is capable of persistent infection in mice but has a clearly reduced capacity to multiply compared to the virulent strain χ3306.
FIG. 3.
Persistence of S. enterica serovar Typhimurium lon disruption mutant in the organs of BALB/c mice following oral administration. A group of five mice was orally inoculated with 5 × 108 CFU of strain CS2022 (lon:: Cmr). On each indicated week after administration, the numbers of bacteria recovered from the spleens, mesenteric lymph nodes (MLN), and Peyer's patches of mice were determined. The error bars indicate the standard deviation the means of these numbers.
Disruption of lon impairs survival of S. enterica serovar Typhimurium in murine macrophages.
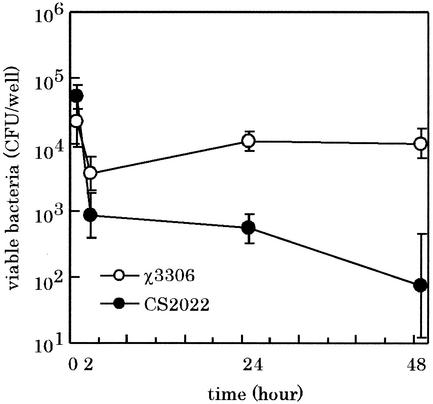
The virulence studies clearly indicated that the S. enterica serovar Typhimurium lon mutant was attenuated for virulence in a murine infection model. One of the most probable factors to reduce the ability of the lon mutant to cause systemic infection is the reduced capacity to survive bactericidal mechanisms of professional killing cells such as macrophages in mouse. To directly address this possibility, the strain CS2022 was assayed to assess its ability to survive and grow in macrophage cells. It is known that S. enterica serovar Typhimurium grown under conditions such as high osmolarity (0.3 M NaCl), oxygen limitation, or change in the pH of the medium from 6.0 to 8.0, which allow expression of the type III secretion system encoded by SPI-1, readily kills cultured macrophage cells (6, 9). To avoid this cytotoxic effect of expression of the type III secretion system, bacterial cells were grown in L-broth (pH 7.4) to challenge the macrophages. The murine macrophage cell line RAW264.7 was challenged with either strain χ3306 or CS2022 for 48 h, and the number of viable bacteria within macrophage cells was determined. As shown in Fig. 4, for strain χ3306, there was a decrease in the number of viable bacteria during the first 2 h, which indicated that the initial interaction with the macrophage was the most bactericidal. After this initial drop, strain χ3306 grew in macrophage cells upon incubation over 48 h. In contrast, strain CS2022 showed a decrease in viability in macrophages over 48 h. Both mutant and parental strains were taken up by macrophages at equivalent levels. These results suggest that the Lon protease is required for the intracellular survival and growth of S. enterica serovar Typhimurium within macrophages.
FIG. 4.
Fate of S. enterica serovar Typhimurium virulent strain and the lon disruption mutant within macrophage cells after phagocytosis. RAW264.7 cells were challenged with the strain χ3306 (lon+) or CS2022 (lon:: Cmr). The numbers of viable bacteria were determined as described in Materials and Methods. The experiment was repeated three times, and the results are shown for a representative experiment. The data are averages of triplicate determinations, and the error bars indicate the standard deviation of the means of these numbers.
S. enterica serovar Typhimurium lon disruption mutant shows increased sensitivity to oxidative and acidic conditions.
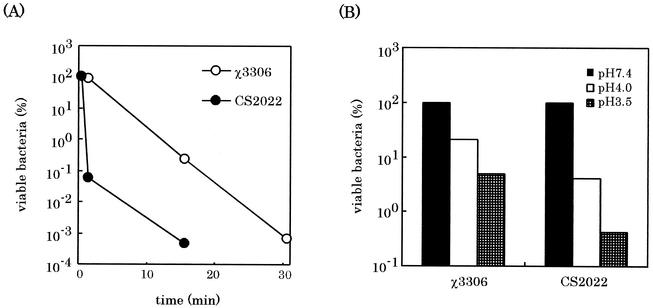
The oxidative-killing mechanism by respiratory burst and nonoxidative killing mechanisms (such as acidic pH in the phagosome and toxic peptides, which are normally sequestered in lysosomes) are known to be associated with digestion of bacteria in macrophages. Therefore, the sensitivity of the lon disruption mutant to hydrogen peroxide which mimics the oxidative killing mechanism was assessed by survival assay after exposing to 15 mM hydrogen peroxide. As shown in Fig. 5A, strain CS2022 was killed more rapidly than the wild type was, suggesting the increased sensitivity of the lon disruption mutant to hydrogen peroxide. Furthermore, the survival of the lon mutant under acidic conditions was examined by incubation for 30 min in the L-broth at pH 4.0 and 3.5 and subsequent counting of the number of viable bacteria. As shown in Fig. 5B, 20 and 5% of the wild-type strain χ3306 inoculum survived in the medium at pH 4.0 and 3.5, respectively, after 30 min. On the other hand, only 4 and 0.4% of the strain CS2022 inoculum survived at pH 4.0 and 3.5, respectively. These results indicate that the Lon protease of S. enterica serovar Typhimurium is required to survive acidic conditions.
FIG. 5.
Effect of lon disruption on sensitivity of S. enterica serovar Typhimurium to oxidative (A) and acidic (B) stresses. To assess the sensitivity to oxidative stress, exponentially growing cells of strains χ3306 (lon+) and CS2022 (lon:: Cmr) were exposed to 15 mM hydrogen peroxide in L-broth at 37°C. After serial dilution at the indicated times, samples were plated on L-agar plates to determine the number of viable cells. To assess the sensitivity to acidic stress, exponentially growing cells in L-broth (pH 7.4) were diluted 1:100 into L-broth at pH 4.0 or 3.5 which were previously adjusted with citric acid and then incubated 37°C. After 30 min, bacterial cells were diluted into L-broth (pH 7.4) and then transferred to the L-agar plate to determine the number of viable cells. Results are represented as a percentage of surviving cells after exposure to acidic stress to the cells present before exposure.
Since Salmonella species are known to be typically resistant to the killing activity of complement that is present in serum (21, 26), we examined whether the lon disruption causes the impaired ability of S. enterica serovar Typhimurium to resist to the mouse serum. Neither wild-type χ3306 nor the mutant CS2022 was found to be sensitive to the killing action of mouse serum (data not shown).
DISCUSSION
Lon protease is a cytoplasmic protein in procaryotes and a mitochondrial matrix protein in eucaryotes. In Saccharomyces cerevisiae, Lon appears to have protein remodeling ability, judging by its in vivo role in membrane assembly (38). In E. coli, Lon has been shown to participate in proteolysis of both specific protein targets and abnormal proteins (16). Specific targets—SulA, a regulator of the SOS response, and RcsA, a transcriptional activator of genes of capsular polysaccharide-have been well characterized (39, 45). Our recent findings proposed a novel target that is a putative factor to be involved in activation of HilA, a central regulator of SPI1 gene expression on the Salmonella chromosome (43). The disruption of lon in S. enterica serovar Typhimurium χ3306 resulted in a dramatic increase in the ability of the bacteria to invade Intestine-407 cells, in the secretion of invasion-associated proteins, and in the expression of invasion genes encoded on the SPI1 (43).
Here we have extended the study of this lon mutant by comparing its virulence to that of the parental strain. Mouse infection studies demonstrated that the ability of lon mutant to cause systemic infection was decreased more than 3 log units compared to that of the virulent parent (Table 1). After oral inoculation, the lon mutant was able to reach extraintestinal sites but unable to proliferate efficiently within the spleen of mice (Fig. 1 and 2). Thus, we conclude that the Lon protease is essentially involved in the lethal systemic infection with S. enterica serovar Typhimurium in mice.
To gain a better understanding of why the lon disruption mutant has lost the ability to cause an overwhelming systemic disease in mice, a variety of virulence properties have been examined. Intramacrophage survival and proliferation have been shown to correlate with S. enterica serovar Typhimurium colonization of the mouse spleen and liver (14). Therefore, we examined the fate of the lon mutant after phagocytosis with macrophage cells and found that the lon mutant lost the ability to survive and proliferate within RAW264.7 macrophage cells (Fig. 4), suggesting that the Lon protease of S. enterica serovar Typhimurium is involved in the withstanding of the killing mechanism of macrophage and in growth intracellularly.
During macrophage killing of bacteria, bacteria are first engulfed by endocytosis into phagosomes, which then fuse with lysosomes to form phagolysosomes. The phagolysosome contains oxygen-dependent and -independent mechanisms for killing the invading bacteria (20, 27, 35). The oxygen-dependent mechanism is based on the high levels of reactive oxygen species, such as hydrogen peroxide and superoxide produced by the respiratory burst, which contribute to the bactericidal capacity of phagocytes. We have shown that the disruption of lon results in the increased sensitivity of S. enterica serovar Typhimurium to hydrogen peroxide (Fig. 5A). Studies on bacterial sensitivity to hydrogen peroxide have been performed to understand the inability of various Salmonella mutants to survive within macrophages (2, 3, 20, 24, 25). Though the bactericidal effect by hydrogen peroxide observed in vitro does not exactly reflect the oxygen-dependent mechanisms for killing the invading bacteria in macrophages, the increased sensitivity of the mutant to hydrogen peroxide would be able to explain partly its impaired ability to survive the phagocytosis.
On the other hand, the oxygen-independent killing mechanism includes acidification of the phagosome, degradation of its contents by proteins with antimicrobial activity associated with the lysosome, and the microbicidal activity of the reactive nitrogen intermediate (10, 20, 27, 35). The increased sensitivity of the lon disruption mutant to acidic pH demonstrated in the present study (Fig. 5B) could partly explain its impaired ability to survive phagocytosis. The Lon protease is known to be a major contributor to the degradation of abnormal proteins in E. coli. Its substrates include heat-damaged proteins, puromycyl proteins, and many missense proteins and nonsense fragments (29, 36, 44). It is probable that acid-damaged proteins are also targeted and eliminated by the Lon protease, although such a function has not been previously proven for Lon protease.
One of the well-known phenotypes of lon mutants is sensitivity to DNA-damaging agents such as UV. We observed that the mutant strain CS2022 is extremely sensitive to UV irradiation, similar to that described in a previous report (12) (data not shown), suggesting that the Lon protease is also involved in the degradation of the emergency response timing protein, similar to SulA in Salmonella. Though the disruption of S. enterica serovar Typhimurium lon gene in the present study resulted in the increased susceptibility of bacteria to various stresses, it did not cause temperature-sensitive growth of bacteria; that is, the mutant did grow as well as the wild type at 37 and 42°C (data not shown).
Taken together, the impaired ability of the lon mutant to survive and grow in macrophage could be due to the enhanced susceptibility to the oxidative killing mechanism associated with respiratory burst and the low phagosomal pH. Recent works on SPI indicated that the genetic element carried on SPI2 has a central role in systemic growth of Salmonella in its host and is required for bacterial proliferation in macrophages (22). At present, we cannot exclude the possibility that the Lon protease directly modulates the levels of the major contributors for virulence specified by the SPI2. Our previous study has demonstrated that the Lon protease negatively regulates the expression of invasion genes carried on the SPI1 (43). Like the PhoP/PhoQ regulatory system, Lon may oppositely modulate two major contributors, epithelial invasion and survival within macrophages, for the expression of pathogenesis of S. enterica serovar Typhimurium.
Most energy-dependent proteolysis in the E. coli cytosol has been attributed to well-characterized proteases: Lon, the Clp family (ClpAP and ClpXP), and HslVU, which are also known as stress proteins (7, 16, 40). We have previously reported that the ClpXP is essentially required for systemic infection of S. enterica serovar Typhimurium in mice (46). In contrast, HslVU protease did not seem to be involved in the pathogenesis of S. enterica serovar Typhimurium (our unpublished data).
Of further interest is the finding that the lon disruption mutant persists in the BALB/c mouse for long periods without causing an overwhelming systemic infection (Fig. 3).
Various S. enterica serovar Typhimurium mutants that cause persistent infection in mice have been described. These include an aro mutant and a pur mutant (4, 5, 28, 34), a cya-crp mutant (8), an ompR mutant (11), an htrA mutant (25), an agfA mutant (41), an surA mutant (42), an rpoS mutant (13), and an rpoE mutant (24). Among them, the mutants for htrA, aro, pur, cya-crp, and surA have demonstrated potential as vaccine candidates, since mice inoculated with these mutants were protected against subsequent challenge with an S. enterica serovar Typhimurium virulent strain. Recently, we have found that mice persistently infected with the lon mutant are protected against subsequent challenge with S. enterica serovar Typhimurium χ3306 (our unpublished data). To assess the potential of the lon disruption mutant as an effective live oral vaccine, further characterization of the mutant strain CS2022 and studies on the chronic carrier state in mice are in progress.
Acknowledgments
This research was supported by grants-in-aid for scientific research to T. Yamamoto (13470058) and to H. Matsui (13670288) from the Ministry of Education, Culture, Sports, Science, and Technology of the Japanese government.
Editor: A. D. O'Brien
REFERENCES
- 1.Bajaj, V., C. Hwang, and C. A. Lee. 1995. hilA is a novel ompR/toxR member that activates the expression of Salmonella typhimurium invasion genes. Mol. Microbiol. 18:715-727. [DOI] [PubMed] [Google Scholar]
- 2.Buchmeier, N., S Bossie, C. Y. Chen, F. C. Fang, D. G. Guiney, and S. J. Libby. 1997. SlyA, a transcriptional regulator of Salmonella typhimurium, is required for resistance to oxidative stress and is expressed in the intracellular environment of macrophages. Infect. Immun. 65:3725-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchmeier, N. A., C. J. Lipps, M. Y. H. So, and F. Heffron. 1993. Recombination-deficient mutants of Salmonella typhimurium are avirulent and sensitive to oxidative burst of macrophages. Mol. Microbiol. 7:933-936. [DOI] [PubMed] [Google Scholar]
- 4.Carter, P. B., and F. M. Collins. 1974. The route of enteric infection in normal mice. J. Exp. Med. 139:1189-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatfield, S. N., K. Strahan, D. Pickard, I. G. Charles, C. E. Hormaeche, and G. Dougan. 1992. Evaluation of Salmonella typhimurium strains harbouring defined mutations in htrA and aroA in the murine salmonellosis model. Microb. Pathog. 12:145-151. [DOI] [PubMed] [Google Scholar]
- 6.Chen, L. M., K. Kaniga, and J. E. Galan. 1996. Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol. 21:1101-1115. [DOI] [PubMed] [Google Scholar]
- 7.Chung, C. H. 1993. Proteases of Escherichia coli. J. Biol. Chem. 262:372-374. [DOI] [PubMed] [Google Scholar]
- 8.Curtis, R., III, and S. M. Kelly. 1987. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect. Immun. 55:3035-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daefler, S. 1999. Type III secretion by Salmonella typhimurium does not require contact with an eukaryotic host. Mol. Microbiol. 31:45-51. [DOI] [PubMed] [Google Scholar]
- 10.De Groote, M. A., D. Granger, Y. Xu, G. Campbell, R. Prince, and F. C. Fang. 1995. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc. Natl. Acad. Sci. USA 92:6399-6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorman, C. J., S. Chatfield, C. F. Higgins, C. Hayward, and G. Dougan. 1989. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect. Immun. 57:2136-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowins, D., L. Waxman, A. L. Goldberg, and J. Roth. 1986. Isolation and characterization of lon mutants in Salmonella typhimurium. J. Bacteriol. 165:193-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang, F. C., S. J. Libby, N. A. Buchmeier, P. C. Loewen, J. Switala, J. Harwood, and D. G. Guiney. 1992. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc. Natl. Acad. Sci. USA 89:11978-11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finlay, B. B. 1994. Molecular and cellular mechanisms of Salmonella pathogenesis. Curr. Top. Microbiol. Immunol. 192:163-185. [DOI] [PubMed] [Google Scholar]
- 16.Gottesman, S. 1996. Proteases and their targets in Escherichia coli. Annu. Rev. Genet. 30:465-506. [DOI] [PubMed] [Google Scholar]
- 17.Groisman, E., and H. Ochman. 1997. How Salmonella became a pathogen. Trends Microbiol. 5:343-349. [DOI] [PubMed] [Google Scholar]
- 18.Gulig, P. A., and R. Curtiss, III. 1987. Plasmid-associated virulence of Salmonella typhimurium. Infect. Immun. 55:2891-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen-Wester, I., and M. Hensel. 2001. Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect. 3:549-559. [DOI] [PubMed] [Google Scholar]
- 20.Hassett, D. J., and M. S. Cohen. 1989. Bacterial adaptation to oxidative stress: implications for pathogenesis and interaction with phagocytic cells. FASEB J. 3:2574-2582. [DOI] [PubMed] [Google Scholar]
- 21.Heffernan, E. J., H. Harwood, J. Fierer, and D. Guiney. 1992. The Salmonella typhimurium virulence plasmid complement resistance gene rck is homologous to a family of virulence-related outer membrane protein genes, including pagC and ail. J. Bacteriol. 174:84-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hensel, M. 2000. Salmonella pathogenicity island 2. Mol. Microbiol. 36:1015-1023. [DOI] [PubMed] [Google Scholar]
- 23.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humphreys, S., A. Stevenson, A. Bacon, A. B. Weinhardt, and M. Roberts. 1999. The alternative sigma factor, σE, is critically important for the virulence of Salmonella typhimurium. Infect. Immun. 67:1560-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, K., I. Charles, G. Dougan, D. Pickard, P. O'Gaora, T. Ali, I. Miller, and C. Hormaeche. 1991. The role of a stress-response protein in Salmonella typhimurium virulence. Mol. Microbiol. 5:401-407. [DOI] [PubMed] [Google Scholar]
- 26.Joiner, K. A. 1988. Complement evasion by bacteria and parasites. Annu. Rev. Microbiol. 42:201-230. [DOI] [PubMed] [Google Scholar]
- 27.Jones, B. D., and S. Falkow. 1996. Salmonellosis: host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 14:533-561. [DOI] [PubMed] [Google Scholar]
- 28.Killar, L. M., and T. K. Eisenstein. 1985. Immunity to Salmonella typhimurium infection in C3H/HeJ and C3H/HeNCrlBR mice: studies with an aromatic-dependent live S. typhimurium strain as a vaccine. Infect. Immun. 47:605-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laskowska, E., D. Kuczynska-Wisnik, and J. Skorko-Glonek, and A. Taylor. 1996. Degradation by proteases Lon, Clp and HtrA, of Escherichia coli proteins aggregated in vivo by heat shock; HtrA protease action in vivo and in vitro. Mol. Microbiol. 22:555-571. [DOI] [PubMed] [Google Scholar]
- 30.Lucas, R. L., and C. A. Lee. 2000. Unravelling the mysteries of virulence gene regulation in Salmonella typhimurium. Mol. Microbiol. 36:1024-1033. [DOI] [PubMed] [Google Scholar]
- 31.Lucas, R. L., C. P. Lostroh, C. C. DiRusso, M. P. Spector, B. L. Wanner, and C. A. Lee. 2000. Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:1872-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mills, D. M., V. Bajaj, and C. A. Lee. 1995. A 40 kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol. Microbiol. 15:749-759. [DOI] [PubMed] [Google Scholar]
- 33.Morimoto, R. I., A. Tissieres, and C. Georgopoulos. 1994. Progress and perspectives on the biology of heat shock proteins and molecular chaperone, p. 1-30. In R. I. Morimoto, A. Tissieres, and C. Georgopoulos (ed.), The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.O'Callaghan, D., D. Maskell, F. Y. Liew, C. S. F. Easmon, and G. Dougan. 1988. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attenuation, persistence, and ability to induce protective immunity in BALB/c mice. Infect. Immun. 56:419-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pacelli, R., D. A. Wink, J. A. Cook, M. C. Krishna, W. DeGraff, N. Friedman, M. Tsokos, A. Samuni, and J. B. Mitchell. 1995. Nitric oxide potentiates hydrogen peroxide-induced killing of Escherichia coli. J. Exp. Med. 182:1469-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parcell, D. A., and S. Lindquist. 1993. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged protein. Annu. Rev. Genet. 27:437-496. [DOI] [PubMed] [Google Scholar]
- 37.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 38.Rep, M., J. M. van Dijl, K. Suda, G. Schatz, L. A. Grivell, and C. K. Suzuki. 1996. Promotion of mitochondrial membrane complex assembly by a proteolytically inactive yeast Lon. Science 274:103-106. [DOI] [PubMed] [Google Scholar]
- 39.Schoemaker, J. M., C. G. Randall, and A. Markovitz. 1984. Regulation of cell division in Escherichia coli: SOS induction and cellular location of the SulA protein, a key to lon-associated filamentation and death. J. Bacteriol. 158:551-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith, C. K., T. A. Baker, and R. T. Sauger. 1999. Lon and Clp family protease and chaperones share homologous substrate-recognition domains. Proc. Natl. Acad. Sci. USA 96:6678-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sukupolvi, S., A. Edelstein, M. Rhen, S. J. Normark, and J. D. Pfeifer. 1997. Development of a murine model of chronic Salmonella infection. Infect. Immun. 65:838-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sydenham, M., G. Douce, F. Bowe, S. Ahmed, S. Chatfield, and G. Dougan. 2000. Salmonella enterica serovar Typhimurium surA mutants are attenuated and effective live oral vaccines. Infect. Immun. 68:1109-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takaya, A., T. Tomoyasu, A. Tokumitsu, M. Morioka, and T. Yamamoto. 2002. The ATP-dependent Lon protease of Salmonella enterica serovar Typhimurium regulates invasion and expression of genes carried on Salmonella pathogenicity island 1. J. Bacteriol. 184:224-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomoyasu, T., A. Mogk, H. Langen, P. Goloubinoff, and B. Bukau. 2001. Genetic dissection of the roles of chaperones and proteases in protein folding and degradation in the Escherichia coli cytosol. Mol. Microbiol. 40:397-413. [DOI] [PubMed] [Google Scholar]
- 45.Torres-Cabassa, A. S., and S. Gottesman. 1987. Capsule synthesis in Escherichia coli K-12 is regulated by proteolysis. J. Bacteriol. 169:981-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto, T., H. Sashinami, A. Takaya, T. Tomoyasu, H. Matsui, T. Hanawa, S. Kamiya, and A. Nakane. 2001. Disruption of the genes for ClpXP protease in Salmonella enterica serovar Typhimurium results in a persistent infection in mouse and development of the persistence requires endogenous Interferon-gamma and tumor necrosis factor-alpha. Infect. Immun. 69:3164-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]