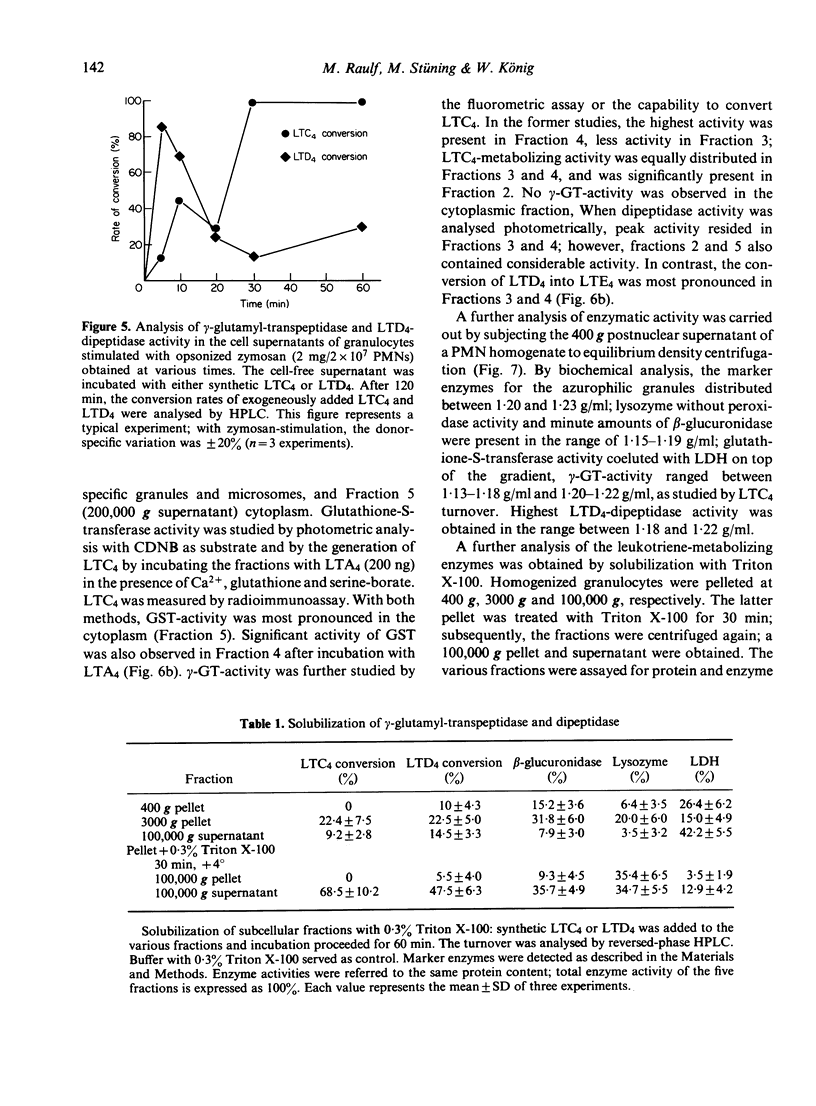
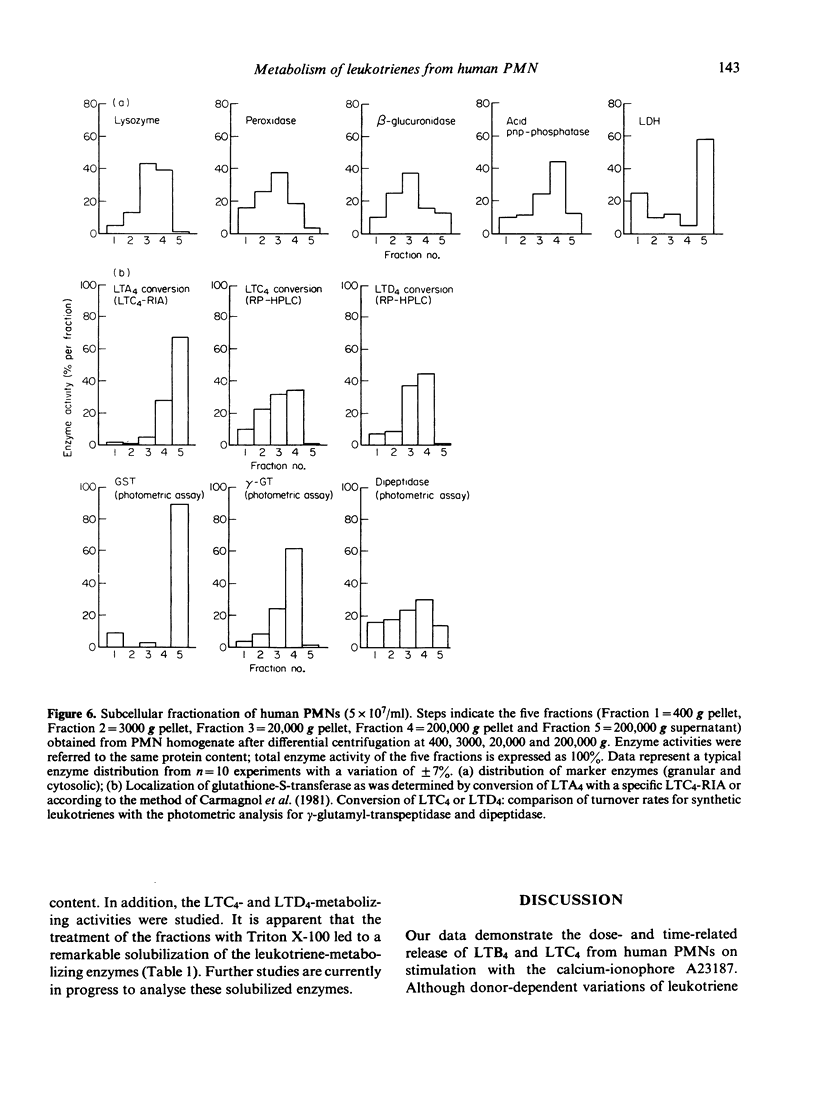
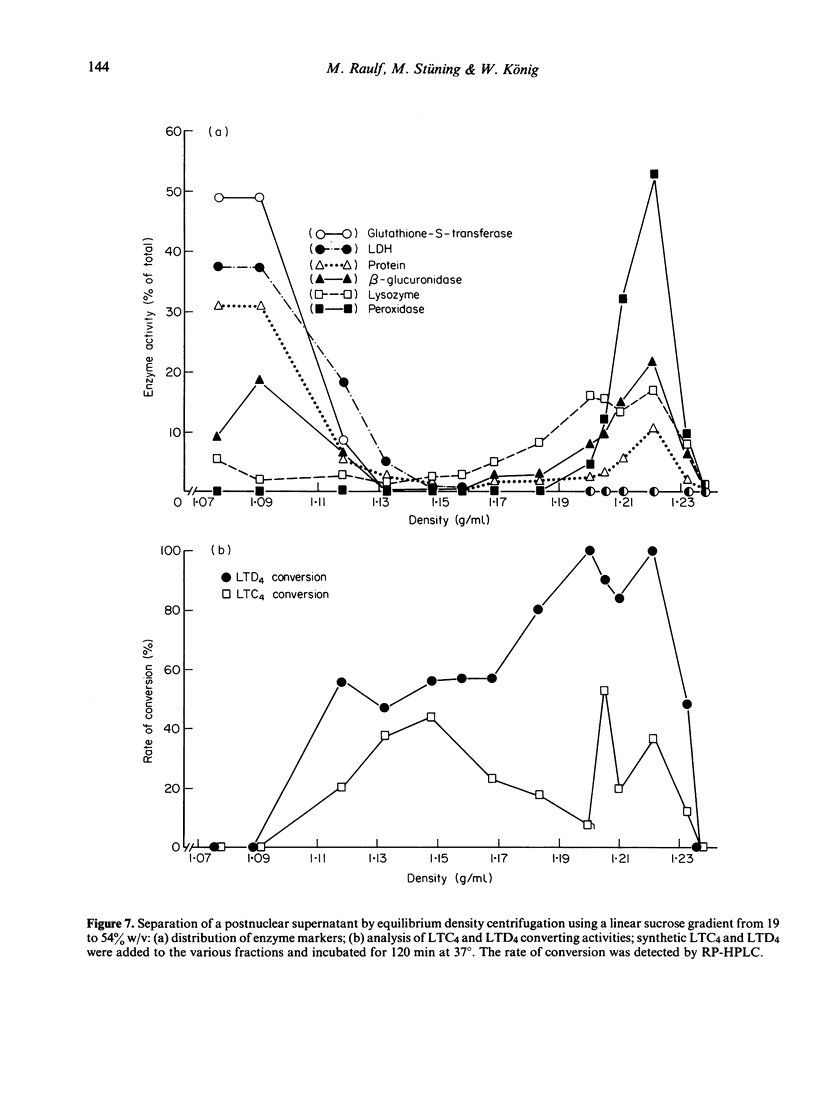
Abstract
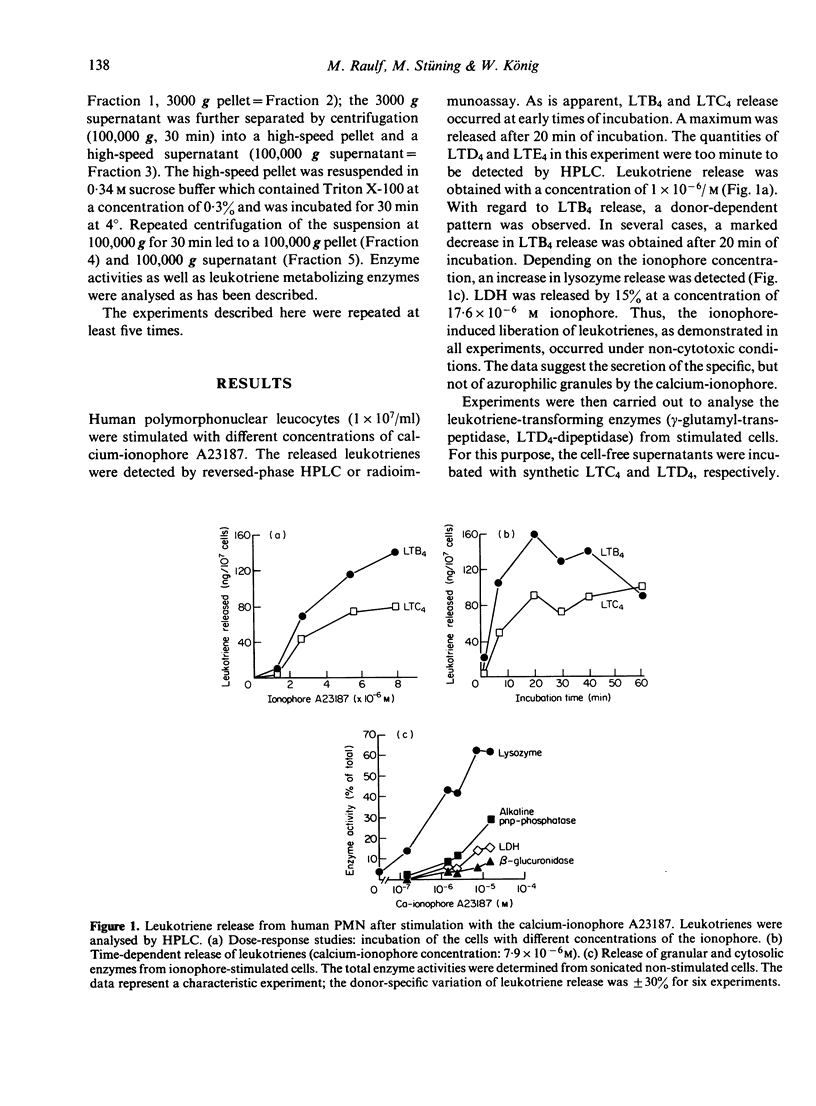
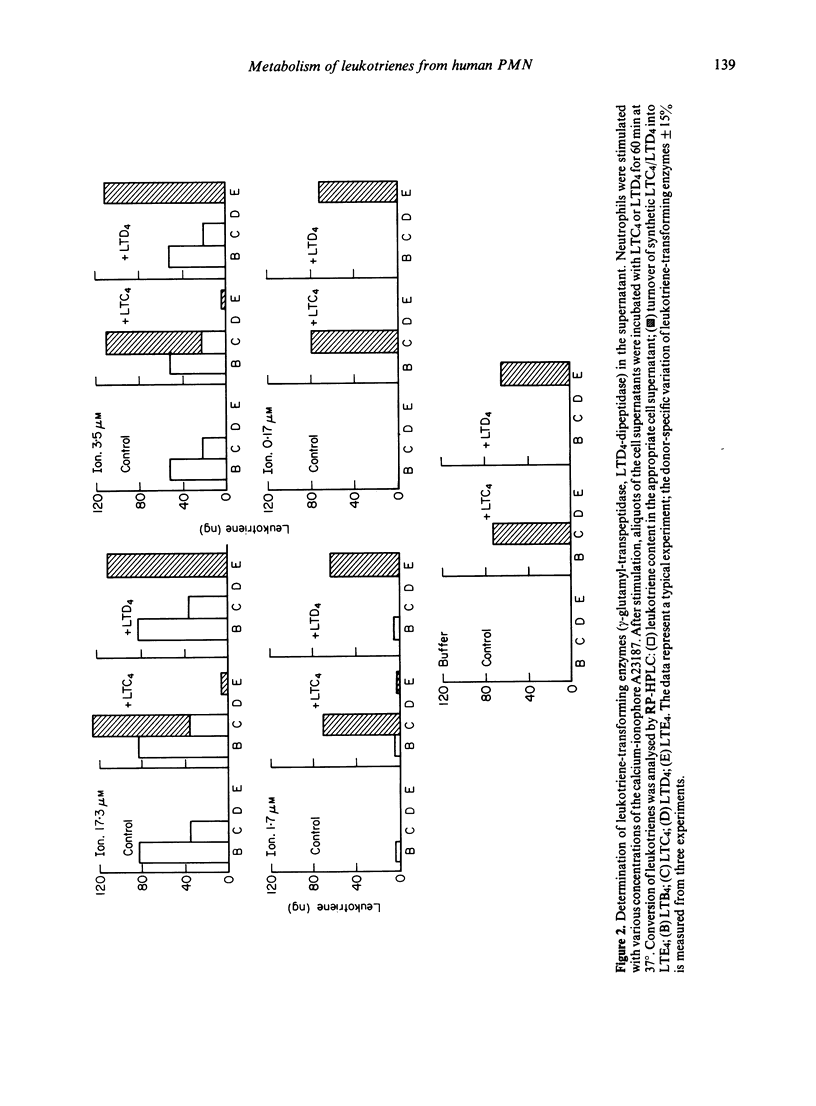
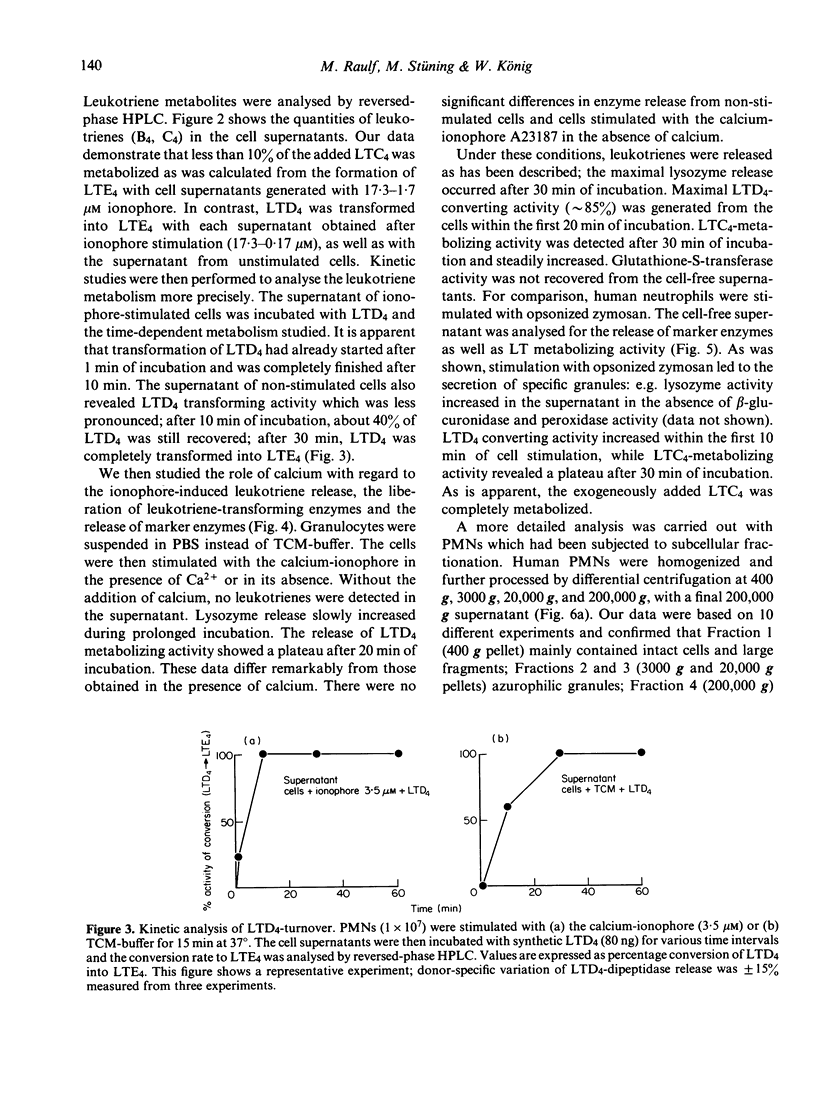
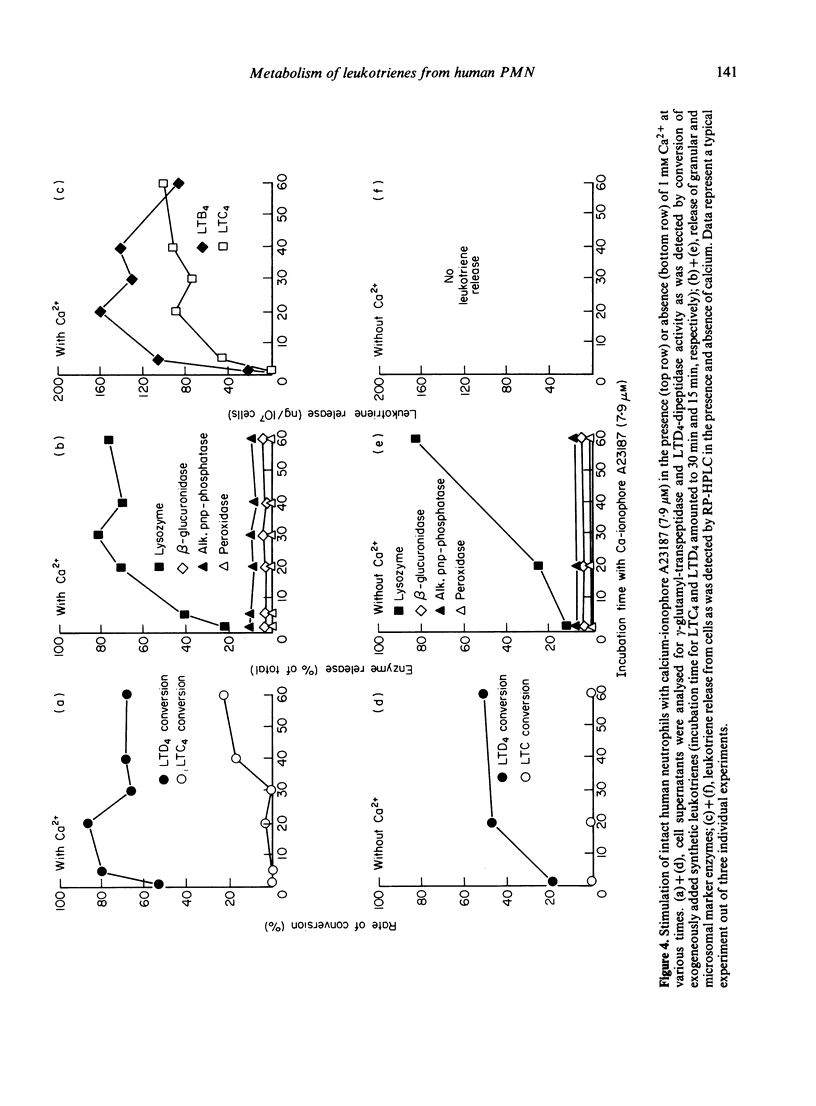
Stimulation of human polymorphonuclear granulocytes with the calcium-ionophore A23187 and opsonized zymosan leads to the release of leukotrienes. The cell-free supernatants of the stimulated cells revealed gamma-glutamyl-transpeptidase and dipeptidase activity which induced the metabolism of exogeneously added LTC4 and LTD4 respectively. No glutathione-S-transferase activity was present in the supernatant. In the absence of calcium, no leukotrienes were generated; dipeptidase activity was slowly released and gamma-glutamyl-transpeptidase activity was not detected. By subcellular fractionation, glutathione-S-transferase activity was present in the microsomal and cytosol fractions, and gamma-glutamyl-transpeptidase and dipeptidase were recovered from the granular and microsomal fractions. By equilibrium density-gradient centrifugation, highest dipeptidase activity eluted in the range between 1.18 and 1.22 g/ml; gamma-glutamyl-transpeptidase was present in the range from 1.13 to 1.18 g/ml and 1.20 to 1.22 g/ml; glutathione-S-transferase did not enter the gradient under these conditions. Solubilization of the 100,000 g pellet of homogenized cells with Triton X-100 led to the release of soluble gamma-glutamyl-transpeptidase and dipeptidase enzymes into the supernatant.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aehringhaus U., Wölbling R. H., König W., Patrono C., Peskar B. M., Peskar B. A. Release of leukotriene C4 from human polymorphonuclear leucocytes as determined by radioimmunoassay. FEBS Lett. 1982 Sep 6;146(1):111–114. doi: 10.1016/0014-5793(82)80715-1. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Allison R. D., Meister A. Interconversion of leukotrienes catalyzed by purified gamma-glutamyl transpeptidase: concomitant formation of leukotriene D4 and gamma-glutamyl amino acids. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1088–1091. doi: 10.1073/pnas.79.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. K., Brashler J. R., Morton D. R., Jr Solubilization and characterization of the leukotriene C4 synthetase of rat basophil leukemia cells: a novel, particulate glutathione S-transferase. Arch Biochem Biophys. 1984 May 1;230(2):455–465. doi: 10.1016/0003-9861(84)90426-0. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: unstable intermediate in formation of dihydroxy acids. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3213–3217. doi: 10.1073/pnas.76.7.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremm K. D., Brom J., König W., Spur B., Crea A., Bhakdi S., Lutz F., Fehrenbach F. J. Generation of leukotrienes and lipoxygenase factors from human polymorphonuclear granulocytes during bacterial phagocytosis and interaction with bacterial exotoxins. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Jul;254(4):500–514. [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brom J., Raulf M., Stüning M., Spur B., Crea A., Bremm K. D., König W. Subcellular localization of enzymes involved in leukotriene formation within human polymorphonuclear granulocytes. Immunology. 1984 Mar;51(3):571–583. [PMC free article] [PubMed] [Google Scholar]
- Carmagnol F., Sinet P. M., Rapin J., Jerome H. Glutathione-S-transferase of human red blood cells; assay, values in normal subjects and in two pathological circumstances: hyperbilirubinemia and impaired renal function. Clin Chim Acta. 1981 Dec 9;117(2):209–217. doi: 10.1016/0009-8981(81)90040-1. [DOI] [PubMed] [Google Scholar]
- Conroy M. C., Orange R. P., Lichtenstein L. M. Release of slow reacting substance of anaphylaxis (SRS-A) from human leukocytes by the calcium ionophore A23187. J Immunol. 1976 Jun;116(6):1677–1681. [PubMed] [Google Scholar]
- Feinmark S. J., Lindgren J. A., Claesson H. E., Malmsten C., Samuelsson B. Stimulation of human leukocyte degranulation by leukotriene B4 and its omega-oxidized metabolites. FEBS Lett. 1981 Dec 21;136(1):141–144. doi: 10.1016/0014-5793(81)81233-1. [DOI] [PubMed] [Google Scholar]
- Frickhofen N., König W. Subcellular localization of the eosinophil chemotactic factor (ECF) and its inactivator in human polymorphonuclear leucocytes (PMN). Immunology. 1979 May;37(1):111–122. [PMC free article] [PubMed] [Google Scholar]
- Friedberg T., Milbert U., Bentley P., Guenther T. M., Oesch F. Purification and characterization of a new cytosolic glutathione S-transferase (glutathione S-transferase X) from rat liver. Biochem J. 1983 Dec 1;215(3):617–625. doi: 10.1042/bj2150617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Horn J. K., Kaplan H. B., Weissmann G. Calcium-induced lysozyme secretion from human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1974 Sep 23;60(2):807–812. doi: 10.1016/0006-291x(74)90312-x. [DOI] [PubMed] [Google Scholar]
- Gzarnetzki B. M., Konig W., Lichtenstein L. M. Release of eosinophil chemotactic factor from human polymorphonuclear neutrophils by calcium ionophore A23187 and phagocytosis. Nature. 1975 Dec 25;258(5537):725–726. doi: 10.1038/258725a0. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Glutathione S-transferases (rat and human). Methods Enzymol. 1981;77:218–231. doi: 10.1016/s0076-6879(81)77029-0. [DOI] [PubMed] [Google Scholar]
- Hammarström S. Leukotrienes. Annu Rev Biochem. 1983;52:355–377. doi: 10.1146/annurev.bi.52.070183.002035. [DOI] [PubMed] [Google Scholar]
- Hammarström S. Metabolism of leukotriene C3 in the guinea pig. Identification of metabolites formed by lung, liver, and kidney. J Biol Chem. 1981 Sep 25;256(18):9573–9578. [PubMed] [Google Scholar]
- Ito Y., Sugiura M., Sawaki S. Purification and properties of human pancreas dipeptidase. J Biochem. 1983 Sep;94(3):871–877. doi: 10.1093/oxfordjournals.jbchem.a134430. [DOI] [PubMed] [Google Scholar]
- Jakschik B. A., Harper T., Murphy R. C. Leukotriene C4 and D4 formation by particulate enzymes. J Biol Chem. 1982 May 25;257(10):5346–5349. [PubMed] [Google Scholar]
- Jakschik B. A., Kuo C. G. Subcellular localization of leukotriene-forming enzymes. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:141–145. [PubMed] [Google Scholar]
- Jörg A., Henderson W. R., Murphy R. C., Klebanoff S. J. Leukotriene generation by eosinophils. J Exp Med. 1982 Feb 1;155(2):390–402. doi: 10.1084/jem.155.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak E. M., Tate S. S. Glutathione-degrading enzymes of microvillus membranes. J Biol Chem. 1982 Jun 10;257(11):6322–6327. [PubMed] [Google Scholar]
- Kroegel C., König W., Mollay C., Kreil G. Generation of the eosinophil chemotactic factor (ECF) from various cell types by melittin. Mol Immunol. 1981 Mar;18(3):227–236. doi: 10.1016/0161-5890(81)90089-4. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., McKinney J. D. Identity of microsomal glutathione S-transferases. Mol Cell Biochem. 1982 Oct 18;48(2):91–96. doi: 10.1007/BF00227609. [DOI] [PubMed] [Google Scholar]
- Meister A., Tate S. S. Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- Meister A., Tate S. S., Griffith O. W. Gamma-glutamyl transpeptidase. Methods Enzymol. 1981;77:237–253. doi: 10.1016/s0076-6879(81)77032-0. [DOI] [PubMed] [Google Scholar]
- Morris H. R., Taylor G. W., Jones C. M., Piper P. J., Samhoun M. N., Tippins J. R. Slow reacting substances (leukotrienes): enzymes involved in their biosynthesis. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4838–4842. doi: 10.1073/pnas.79.16.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orning L., Hammarström S. Inhibition of leukotriene C and leukotriene D biosynthesis. J Biol Chem. 1980 Sep 10;255(17):8023–8026. [PubMed] [Google Scholar]
- Orning L., Hammarström S. Kinetics of the conversion of leukotriene C by gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1304–1309. doi: 10.1016/0006-291x(82)91255-4. [DOI] [PubMed] [Google Scholar]
- Patterson E. K. A dipeptidase from Escherichia coli B. Methods Enzymol. 1976;45:377–386. doi: 10.1016/s0076-6879(76)45033-4. [DOI] [PubMed] [Google Scholar]
- Powell W. S. Properties of leukotriene B4 20-hydroxylase from polymorphonuclear leukocytes. J Biol Chem. 1984 Mar 10;259(5):3082–3089. [PubMed] [Google Scholar]
- Rådmark O., Malmsten C., Samuelsson B., Clark D. A., Goto G., Marfat A., Corey E. J. Leukotriene A: stereochemistry and enzymatic conversion to leukotriene B. Biochem Biophys Res Commun. 1980 Feb 12;92(3):954–961. doi: 10.1016/0006-291x(80)90795-0. [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Korchak H. M., Weissmann G. PGBX, a prostagandin derivative, mimics the action of the calcium ionophore A23187 on human neutrophils. J Immunol. 1980 Nov;125(5):2020–2024. [PubMed] [Google Scholar]
- Sirois P., Brousseau Y. Leukotriene transformation by guinea-pig lungs. Prostaglandins Leukot Med. 1983 Feb;10(2):133–143. doi: 10.1016/s0262-1746(83)80004-3. [DOI] [PubMed] [Google Scholar]
- Smith G. D., Ding J. L., Peters T. J. A sensitive fluorimetric assay for gamma-glutamyl transferase. Anal Biochem. 1979 Nov 15;100(1):136–139. doi: 10.1016/0003-2697(79)90122-2. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Ignarro L. J. Bioregulation of lysosomal enzyme secretion from human neutrophils: roles of guanosine 3':5'-monophosphate and calcium in stimulus-secretion coupling. Proc Natl Acad Sci U S A. 1975 Jan;72(1):108–112. doi: 10.1073/pnas.72.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sok D. E., Pai J. K., Atrache V., Sih C. J. Characterization of slow reacting substances (SRSs) of rat basophilic leukemia (RBL-1) cells: effect of cysteine on SRS profile. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6481–6485. doi: 10.1073/pnas.77.11.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S. S., Meister A. Serine-borate complex as a transition-state inhibitor of gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4806–4809. doi: 10.1073/pnas.75.10.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabucchi G., Soranzo M. R., Rossi F. Exocytosis in human polymorphonuclear leukocytes induced by A 23187 and calcium. FEBS Lett. 1975 Jun 1;54(1):44–48. doi: 10.1016/0014-5793(75)81064-7. [DOI] [PubMed] [Google Scholar]



