Abstract
We employed gentamicin-sensitive and -resistant derivatives of Escherichia coli in a macrophage phagocytosis assay that compared λ bacteriophage and gentamicin as extracellular bactericidal agents. Colony counts and direct microscopic examination of phagocytized E. coli supported the conclusion that gentamicin entered macrophages, even at low concentrations, and contributed to their bactericidal activity. Also, two E. coli strains differing in the ability to express the adhesin of type 1 pili (FimH) were distinguishably different in intracellular survival when λ was used as the extracellular killing agent but were indistinguishable when gentamicin was employed.
Much of what we know about the interaction of bacteria and eucaryotic cells in vitro involves the seemingly straightforward assessment of whether a bacterium is located inside or outside of the eucaryotic cell. Various methods have been used to detect, reduce, or eliminate extracellular bacteria. Such methods include direct microscopic evaluation (11) and the use of bacteriostatic antibiotics (10) and lytic bacteriophage (6, 16). The most recent and widely used method has been to measure the degree to which the eucaryotic cell offers protection from the bactericidal effects of gentamicin (7). Aminoglycoside antibiotics such as gentamicin penetrate eucaryotic cells poorly, making it possible to kill extracellular bacteria without affecting internalized bacteria (20).
Whereas the gentamicin protection assay is simple and highly sensitive (7), several reports have indicated that gentamicin is capable of entering macrophages and killing intracellular bacteria (4, 8, 13). Such observations have led to the practice of using low gentamicin concentrations (e.g., 5 μg/ml) and/or keeping exposure to gentamicin as brief as possible. However, such practices have not allayed concerns that the use of this antibiotic has an impact on conclusions about the ability of a particular species of bacteria, or specific mutant derivatives, to survive intracellularly. Nevertheless, no specific instance has been described in which results were materially changed by using an alternative to gentamicin.
In the present study, we utilized an alternative extracellular bactericidal agent (bacteriophage λ) in macrophage phagocytosis assays to show that gentamicin, even at a low concentration, enters and aids macrophages in killing internalized bacteria. Further, we document an instance in which the two methods of extracellular elimination led to different conclusions about the significance of an Escherichia coli adherence organelle in effecting intracellular survival.
Bacterial strains and media.
All of the bacterial strains used were E. coli K-12 derivatives. Lambda-sensitive E. coli strains ORN175 (FimH+) (10) and ORN204 (FimH−) (9) were each genetically marked by selecting versions capable of utilizing mannitol (Mtl+), creating strains ORN222 and ORN223, respectively. In some pilot experiments, λ-resistant versions of strains ORN175 and ORN204 (ORN115 and ORN133, respectively [14]) were additionally utilized. A gentamicin-resistant version of strain ORN223 (ORN224) was obtained by the isolation of a spontaneous gentamicin-resistant mutant. A clear-plaque mutant of bacteriophage λ (λcI71) was a kind gift of A. D. Kaiser. Lambda lysates were prepared and concentrated, and their titers were determined by standard techniques (1, 3, 15). The agar media used were L agar, MinA agar (12), and tetrazolium agar (18).
Lambda phage bactericidal activity.
Pilot experiments revealed that λcΙ71, at concentrations of ca. 109 PFU/ml and greater, eliminated 99.6% ± 0.8% of λ-sensitive bacteria within 0.5 h of addition (but had no effect on λ-resistant E. coli strains) under the conditions employed in our bactericidal assays (9). FimH+ and FimH− E. coli strains were equally susceptible to λ-mediated killing. Lambda titers were completely stable in the presence or absence of macrophages for at least 5 h, and the presence of λ had no effect on macrophage bactericidal ability. This latter point was established in experiments in which macrophages ingested a λ-resistant strain in the presence or absence of λ (gentamicin served as the extracellular killing agent in these experiments). Lambda antiserum (produced in rabbits by standard techniques and having a neutralization constant for λcI71 of approximately 21 min−1) was effective in preventing λ-mediated killing of λ-sensitive E. coli and had no effect on bacterial viability.
On the basis of the foregoing results, we developed a protocol with λ as an extracellular killing agent that was essentially the same as one we had previously developed with gentamicin (9). The only modification of the procedure when λ was employed was the addition of λ antiserum 2 min prior to lysis of macrophages with Triton X-100. The antiserum was necessary to prevent the extracellular λ from infecting the released bacteria and was effective in preventing λ-mediated killing of λ-sensitive E. coli. The antiserum had no adverse effect on λ-resistant E. coli or macrophages at the concentration employed (0.5 μl of undiluted antiserum per ml).
Gentamicin and λ as extracellular bactericidal agents.
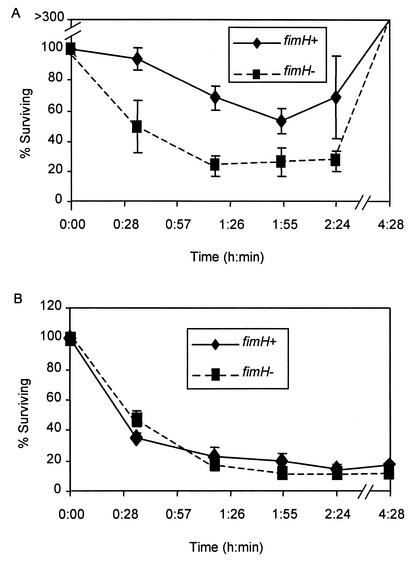
We examined several parameters of macrophage killing curves to determine the degree to which an internalized bacterial population (defined as the population of macrophage-bound bacteria that had survived the 30-min exposure to λ or gentamicin) was reduced. From inspection of compiled data from four killing curves (Fig. 1), it is readily apparent that macrophage bactericidal effectiveness was influenced by the choice of extracellular killing agents (compare Fig. 1A and B). However, to quantitatively assess the effects of the two extracellular killing agents and the effect of the FimH phenotype on macrophage bactericidal activity, a number of curve parameters were examined. These parameters included (i) the initial killing rate of internalized bacteria, (ii) the maximal percentage of internalized bacteria killed, and (iii) the percentage of internalized bacteria that survived the ca. 4.5 h of exposure to the macrophages and the extracellular killing agent.
FIG. 1.
Elimination of internalized FimH+ or FimH− E. coli by resident BALB/c peritoneal macrophages when λ (A) or gentamicin (B) was used as an extracellular killing agent. The FimH+ strains were mixed (1:1) with the FimH− strains and incubated with macrophages. See the text and Table 1, footnotes a to c, for descriptions of the specific bacterial strains employed and the curve parameters compared. Values on the y axis indicate the percentage of internalized bacteria surviving at the times indicated along the x axis. One hundred percent defines that population of macrophage-bound bacteria that survived the 30-min treatment with the extracellular agent. The values shown are averages of four experiments, each performed in duplicate. Error bars denote standard errors of the means. The time points denoted are approximate to allow values from separate experiments to be averaged.
When measuring the initial killing rate (the slope of a line tracing the decrease in the percentage of the internalized E. coli over the first ca. 1.25 h postinternalization), we noted that the rate of FimH− E. coli elimination was significantly greater than that of FimH+ E. coli elimination when λ was used in the assay but the FimH phenotype had no effect when gentamicin was employed (Table 1). With regard to the maximal number of internalized E. coli cells eliminated (the lowest point in the elimination curve) when gentamicin was employed, the magnitude of the decrease was significantly greater than when λ was employed and there was no difference between the maximal elimination levels of FimH+ and FimH− E. coli. When λ was employed, a significantly higher percentage of internalized FimH+ bacteria survived (compared to FimH− bacteria) (Table 1). The last feature of the killing assay examined was the number of bacteria that survived the ca. 4.5 h of exposure to the macrophages and the extracellular killing agent. We noted a pronounced difference in the effects of the extracellular killing agents upon the survival of bacteria that was independent of the FimH phenotype. With λ, the percentage of the protected population present at the end of the experiment increased; with gentamicin, it decreased (Table 1).
TABLE 1.
Summary of the effects of λ and gentamicin as extracellular killing agents on FimH+ or FimH− E. coli bound to resident mouse peritoneal macrophagesa
| Strain | Initial bactericidal rateb
|
Maximum % of internalized bacteria killedc
|
% of internalized bacteria surviving after 4 hc
|
|||
|---|---|---|---|---|---|---|
| λ | Gentamicin | λ | Gentamicin | λ | Gentamicin | |
| FimH+ | 3.5 ± 1.1d,e | 10.9 ± 0.5e | 45.2 ± 7.0d,e | 94.6 ± 1.4e | 548 ± 181e | 4.4 ± 1.3e |
| FimH− | 8.3 ± 1.2d | 10.9 ± 0.4e | 68 ± 5.6d,e | 96.3 ± 1.0e | 692 ± 275e | 2.5 ± 0.4e |
FimH+ strains ORN175 and ORN222 were mixed with their FimH− counterparts (strains ORN204 and ORN223) and employed in BALB/c peritoneal macrophage binding and bactericidal assays as described in the text. All values represent the averages (± standard deviation) of at least four experiments performed in duplicate. Statistically significant differences between means were determined by Student's t test. P < 0.05.
The initial bactericidal rate was measured as the percentage of internalized bacteria eliminated per 10 min as calculated from the negative slope of a regression line through at least three time points covering the first three assay time points (ca 1.25 h). Larger values indicate a more rapid decrease.
The maximal percentage of internalized bacteria killed and the percentage of internalized bacteria surviving after 4 h are expressed as percentages of the internalized population. Values greater than 100% reflect growth of the internalized bacteria.
Statistically significant difference between the FimH+ and FimH− samples with the same extracellular killing agent.
Statistically significant difference between λ and gentamicin as extracellular killing agents with the same bacterial sample.
Gentamicin contributes to macrophage bactericidal effectiveness.
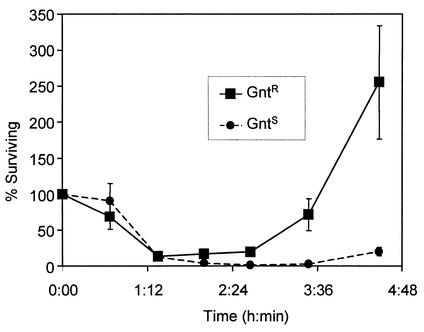
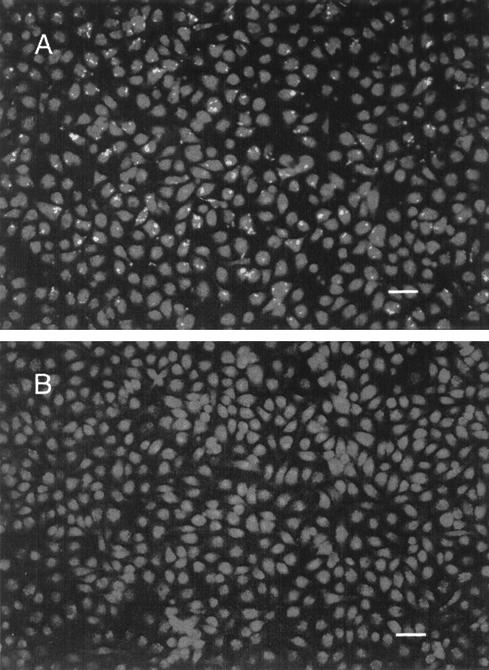
Of the two strains tested, FimH− E. coli appeared to be more susceptible to macrophage killing (i.e., the initial rate of the FimH− bacterial decrease was pronounced regardless of the extracellular killing agent employed [Fig. 1]). However, even with FimH− E. coli, the overall elimination of bacteria, as measured by survival after ca. 4 h, was decidedly more effective with gentamicin. We conducted an experiment to see if resistance to gentamicin had any effect upon the survival of the internalized population, reasoning that if gentamicin was not contributing to the killing of internalized bacteria, then gentamicin resistance should not be a factor in survival. Results affirmed that gentamicin resistance influenced the ability of ingested FimH− E. coli to proliferate when both gentamicin and λ were used as extracellular bactericidal agents (Fig. 2). This effect was most apparent at later times in the assay and was consistent with the idea that gentamicin entered macrophages gradually and contributed to the decrease in intracellular bacteria. This interpretation was supported by microscopic examination of macrophages after 4 h of exposure to FimH+ E. coli and employing either λ or gentamicin as the extracellular killing agent (Fig. 3). The numbers of bacteria in macrophages when λ was used as an extracellular killing agent was particularly striking compared to the dearth of bacteria (both internal and external) when gentamicin was used as the extracellular killing agent (Fig. 3B). The same results were obtained when a FimH− strain (ORN204) was employed but because of the somewhat lower adherence rate of FimH− E. coli (9), more fields needed to be examined (data not shown).
FIG. 2.
Effect of gentamicin resistance upon survival of internalized FimH− E. coli in the presence of extracellular gentamicin and λ. Gentamicin-sensitive (GntS) strain ORN204 was mixed (1:1) with ORN224, a gentamicin-resistant (GntR) mutant of ORN223, and incubated with macrophages as described in the text. Internalized bacteria were defined as those macrophage-associated bacteria that were viable after a 30-min exposure to λ and gentamicin. Bacterial numbers, determined at subsequent time points by plating on mannitol tetrazolium agar, are expressed as a percentage of the internalized population. Each experiment was performed in duplicate. The points represent averages of two separate experiments. The vertical bars indicate standard errors.
FIG. 3.
Microscopic examination of macrophage monolayers containing FimH+ strain ORN175 after 4 h of exposure to λ (A) or gentamicin (B). Macrophage monolayers, obtained as described in the text, were infected and washed, and medium containing either λ or gentamicin was added for the remainder of the experiment. The medium was then removed, and the monolayers were stained with acridine orange-crystal violet as described by Miliotis (11). Viable intracellular bacteria are visible as bright points in panel A. Bar, 40 μm.
Conclusions.
A variety of in vitro assays have been designed to differentiate bacteria that are inside host cells from those that are outside host cells. Gentamicin protection is widely viewed as synonymous with host cell internalization. However, both early (4, 5) and more recent (8, 13, 19) reports of gentamicin leakage into cultured eucaryotic cells have led to the practice of using 5 μg of gentamicin per ml to eliminate extracellular bacteria or using higher concentrations for a brief period, followed by extended periods of incubation with 5 μg of gentamicin per ml. With our detection methods, gentamicin had an effect upon intracellular bacterial viability even at 5 μg/ml.
Our results also demonstrated how the choice of extracellular killing agent can influence conclusions about bacterial factors that may be important for intracellular survival. In previous reports, we and others have utilized different extracellular killing agents to examine the effect of the type 1 pilus ligand (FimH) upon the intracellular fate of E. coli taken up by macrophages via that adhesin (2, 9, 10). The reports all indicate that binding via type 1 pili is not a disadvantage to the bacterium, as one might have expected. However, support for the notion that pili actually facilitate survival of E. coli bound to macrophages (over that observed for bound but nonpiliated E. coli) has been inconsistent (9, 10). Our present results suggest that the choice of extracellular bactericidal agent may be one important variable. Recent experiments with mast cells indicate that internalized FimH+ bacteria survive better than FimH− bacteria because of their direction to different intracellular compartments that differ in the ability to eliminate the bacteria (17). The ability to detect such differences depends, in part, upon the degree to which the killing effected by the cultured cell can be separated from artifactual killing due to gentamicin leakage.
Most generally, our work indicates that if alternatives to gentamicin are available, their use may provide contrasting and informative results.
Acknowledgments
This work was supported by grants AI22223 and DK34987 from the National Institutes of Health and by the State of North Carolina.
Editor: B. B. Finlay
REFERENCES
- 1.Arber, W., L. Enquist, B. Hohn, N. E. Murray, and K. Murray. 1983. Experimental methods for use with λ, p. 433-466. In R.W. Hendrix, J. W. Roberts, F. W. Stahl and R. A. Weisberg (ed.), Lambda II. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 2.Baorto, D. M., Z. Gao, R. Malaviya, M. L. Dustin, A. van der Merwe, D. M. Lublin, and S. N. Abraham. 1997. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature 389:636-639. [DOI] [PubMed] [Google Scholar]
- 3.Davis, R. W., D. Botstein, and J. R. Roth. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 4.Drevets, D. A., B. P. Canono, P. J. Leenen, and P. A. Campbell. 1994. Gentamicin kills intracellular Listeria monocytogenes. Infect. Immun. 62:2222-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edelson, P. J., R. Zwiebel, and Z. A. Cohn. 1975. The pinocytic rate of activated macrophages. J. Exp. Med. 142:1150-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eissenberg, L. G., and P. B. Wyrick. 1981. Inhibition of phagolysosome fusion is localized to Chlamydia psittaci-laden vacuoles. Infect. Immun. 32:889-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsinghorst, E. A. 1994. Measurement of invasion by gentamicin resistance. Methods Enzymol. 236:405-420. [DOI] [PubMed] [Google Scholar]
- 8.Eze, M. O., L. Yuan, R. M. Crawford, C. M. Paranavitana, T. L. Hadfield, A. K. Bhattacharjee, R. L. Warren, and, D. L. Hoover. 2000. Effects of opsonization and gamma interferon on growth of Brucella melitensis 16M in mouse peritoneal macrophages in vitro. Infect. Immun. 68:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamrick, T. S., E. A. Havell, J. R. Horton, and P. E. Orndorff. 2000. Host and bacterial factors involved in the innate ability of mouse macrophages to eliminate internalized unopsonized Escherichia coli. Infect. Immun. 68:125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keith, B. R., S. L. Harris, P. W. Russell, and P. E. Orndorff. 1990. Effect of type 1 piliation on in vitro killing of Escherichia coli by mouse peritoneal macrophages. Infect. Immun. 58:3448-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miliotis, M. 1991. Acridine orange stain for determining intracellular enteropathogens in HeLa cells. J. Clin. Microbiol. 29:830-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 13.Ohya, S., H. Xiong, Y. Tanabe, M. Arakawa, and M. Mitsuyama. 1998. Killing mechanism of Listeria monocytogenes in activated macrophages as determined by an improved assay system. J. Med. Microbiol. 47:211-215. [DOI] [PubMed] [Google Scholar]
- 14.Russell, P. W., and P. E. Orndorff. 1992. Lesions in two Escherichia coli type 1 pilus genes alter pilus number and length without affecting receptor binding. J. Bacteriol. 174:5923-5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 16.Shaw, D. R., A. T. Maurelli, J. D. Goguen, S. C. Straley, and R. Curtiss. 1983. Use of UV-irradiated bacteriophage T6 to kill extracellular bacteria in tissue culture infectivity assays. J. Immunol. Methods 56:75-83. [DOI] [PubMed] [Google Scholar]
- 17.Shin, J. S., Z. Gao, and S. N. Abraham. 2000. Involvement of cellular caveolae in bacterial entry into mast cells. Science 289:785-788. [DOI] [PubMed] [Google Scholar]
- 18.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 19.Tsang, A. W., K. Oestergaard, J. T. Myers, and J. A. Swanson. 2000. Altered membrane trafficking in activated bone marrow-derived macrophages. J. Leukoc. Biol. 68:487-494. [PubMed] [Google Scholar]
- 20.Vaudaux, P., and F. A. Waldvogel. 1979. Gentamicin antibacterial activity in the presence of human polymorphonuclear leukocytes. Antimicrob. Agents Chemother. 16:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]