Abstract
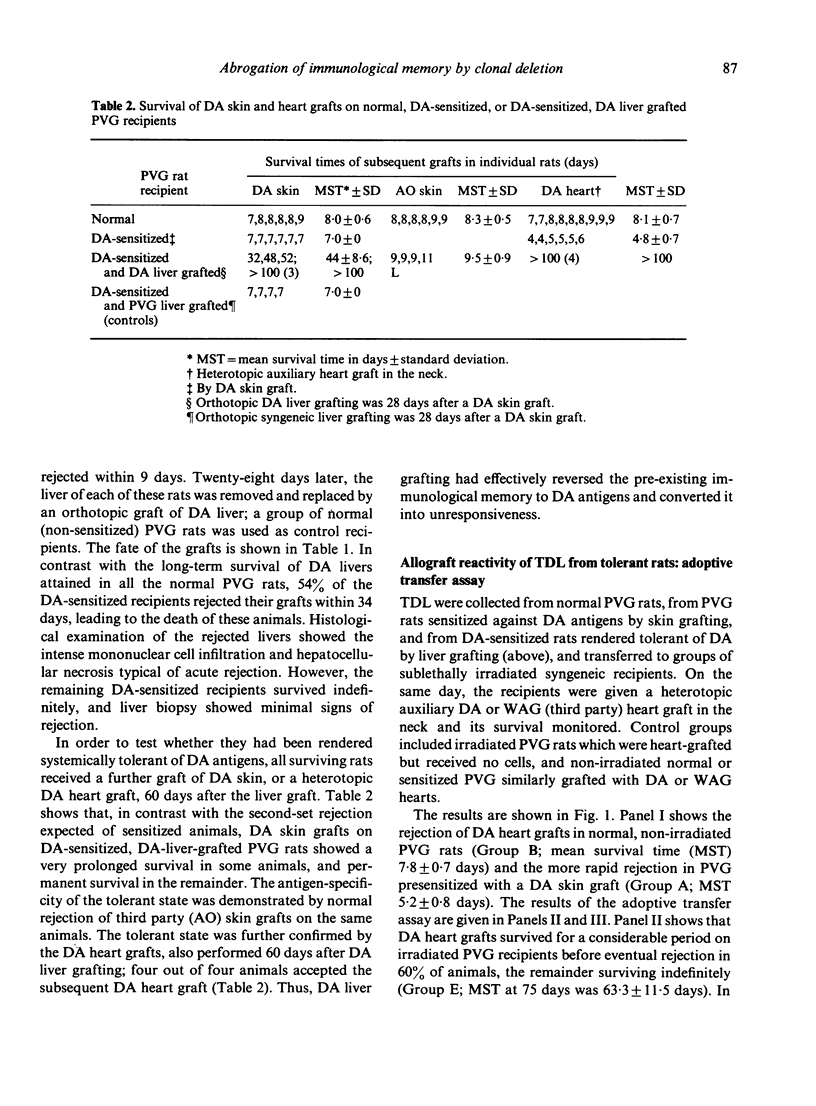
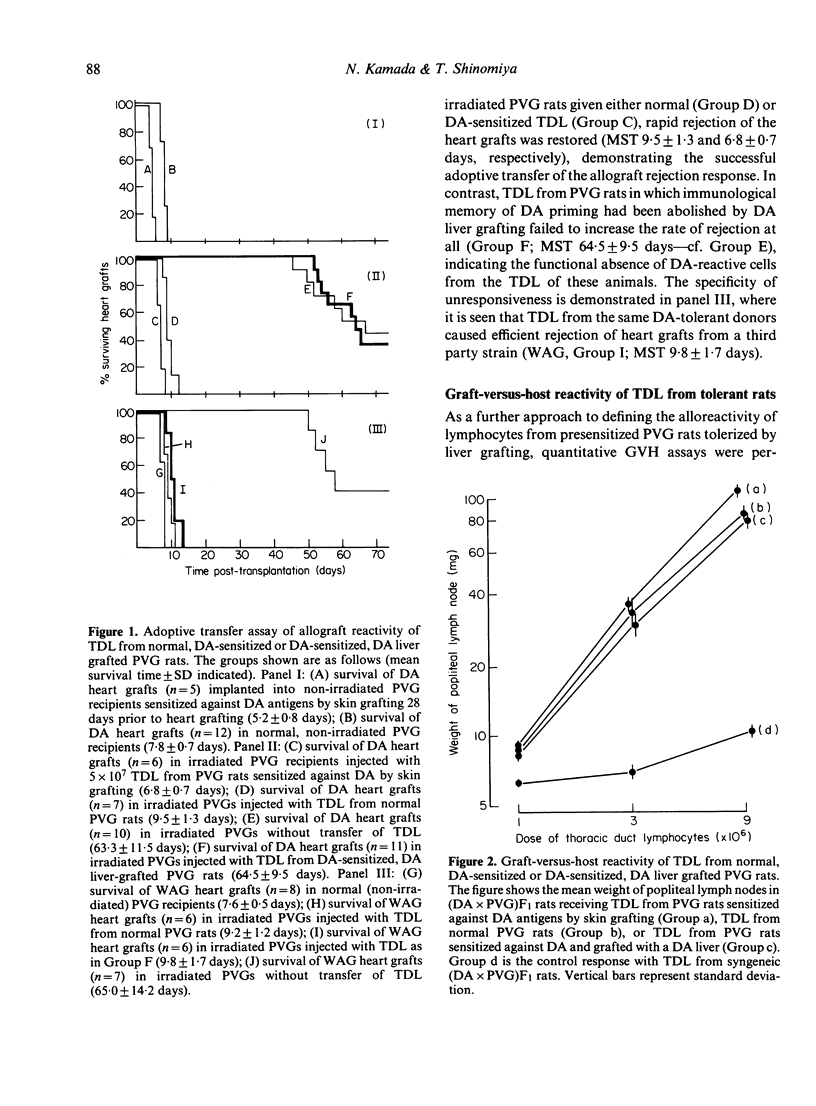
In the rat strain combination of DA into PVG, an orthotopic liver graft has the ability to abrogate an existing state of sensitization against donor (DA) antigens. Fifty-four percent of PVG rats sensitized against DA by skin grafting accepted a DA liver graft permanently, and about half of these became systemically tolerant of DA MHC antigens, as demonstrated by permanent acceptance of a subsequent (second-set) DA skin or heart graft. The cellular basis of this tolerant state was studied in vivo. An adoptive transfer assay provided evidence for functional deletion of DA-reactive cells responsible for graft rejection from the recirculating lymphocyte pool. There was no evidence of a role for suppressor T cells in maintaining tolerance. However, a graft-versus-host assay showed normal reactivity in thoracic duct lymphocytes from tolerant animals. Hence, specific clonal deletion is apparently responsible for the abolition of immunological memory by liver grafting, but is selective in respect of the sets of alloreactive lymphocytes affected.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calne R. Y., Sells R. A., Pena J. R., Davis D. R., Millard P. R., Herbertson B. M., Binns R. M., Davies D. A. Induction of immunological tolerance by porcine liver allografts. Nature. 1969 Aug 2;223(5205):472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- Calne R. Y., White H. J., Yoffa D. E., Maginn R. R., Binns R. M., Samuel J. R., Molina V. P. Observations of orthotopic liver transplantation in the pig. Br Med J. 1967 May 20;2(5550):478–480. doi: 10.1136/bmj.2.5550.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsch S., Roser B. The adoptive transfer of first-set allograft responses by recirculating small lymphocytes in the rat. Aust J Exp Biol Med Sci. 1974 Feb;52(1):33–44. doi: 10.1038/icb.1974.3. [DOI] [PubMed] [Google Scholar]
- Ford W. L., Burr W., Simonsen M. A lymph node weight assay for the graft-versus-host activity of rat lymphoid cells. Transplantation. 1970 Sep;10(3):258–266. doi: 10.1097/00007890-197009000-00007. [DOI] [PubMed] [Google Scholar]
- Galmarini D., Vercesi G., Fassati L. R., Montemagno M., Tarenzi L., Beffagna B., Puricelli C., Pisani F., Pucci C., Farina L. The value of skin allografts in the evaluation of the rejection of liver allotransplants in pigs. Eur Surg Res. 1971;3(5):340–347. doi: 10.1159/000127573. [DOI] [PubMed] [Google Scholar]
- Hall B. M., Dorsch S. E. Cells mediating allograft rejection. Immunol Rev. 1984;77:31–59. doi: 10.1111/j.1600-065x.1984.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Hall B. M., Dorsch S., Roser B. The cellular basis of allograft rejection in vivo. II. The nature of memory cells mediating second set heart graft rejection. J Exp Med. 1978 Oct 1;148(4):890–902. doi: 10.1084/jem.148.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D. N., Fabre J. W. Quantitative studies on the tissue distribution of Ia and SD antigens in the DA and Lewis rat strains. Transplantation. 1979 Feb;27(2):110–119. doi: 10.1097/00007890-197902000-00009. [DOI] [PubMed] [Google Scholar]
- Heron I. A technique for accessory cervical heart transplantation in rabbits and rats. Acta Pathol Microbiol Scand A. 1971;79(4):366–372. doi: 10.1111/j.1699-0463.1971.tb01833.x. [DOI] [PubMed] [Google Scholar]
- Houssin D., Gigou M., Franco D., Bismuth H., Charpentier B., Lang P., Martin E. Specific transplantation tolerance induced by spontaneously tolerated liver allograft in inbred strains of rats. Transplantation. 1980 May;29(5):418–419. doi: 10.1097/00007890-198005000-00015. [DOI] [PubMed] [Google Scholar]
- Kamada N., Brons G., Davies H. S. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation. 1980 May;29(5):429–431. doi: 10.1097/00007890-198005000-00021. [DOI] [PubMed] [Google Scholar]
- Kamada N., Davies H. S., Roser B. J. Fully allogeneic liver grafting and the induction of donor-specific unreactivity. Transplant Proc. 1981 Mar;13(1 Pt 2):837–841. [PubMed] [Google Scholar]
- Kamada N., Davies H. S., Roser B. Reversal of transplantation immunity by liver grafting. Nature. 1981 Aug 27;292(5826):840–842. doi: 10.1038/292840a0. [DOI] [PubMed] [Google Scholar]
- Kamada N., Davies H. S., Wight D., Culank L., Roser B. Liver transplantation in the rat. Biochemical and histological evidence of complete tolerance induction in non-rejector strains. Transplantation. 1983 Apr;35(4):304–311. [PubMed] [Google Scholar]
- Roser B., Ford W. L. Prolonged lymphocytopenia in the rat. the immunological consequences of lymphocyte depletion following injection of 185 W tungsten trioxide into the spleen of lymph nodes. Aust J Exp Biol Med Sci. 1972 Apr;50(2):185–198. [PubMed] [Google Scholar]



