Abstract
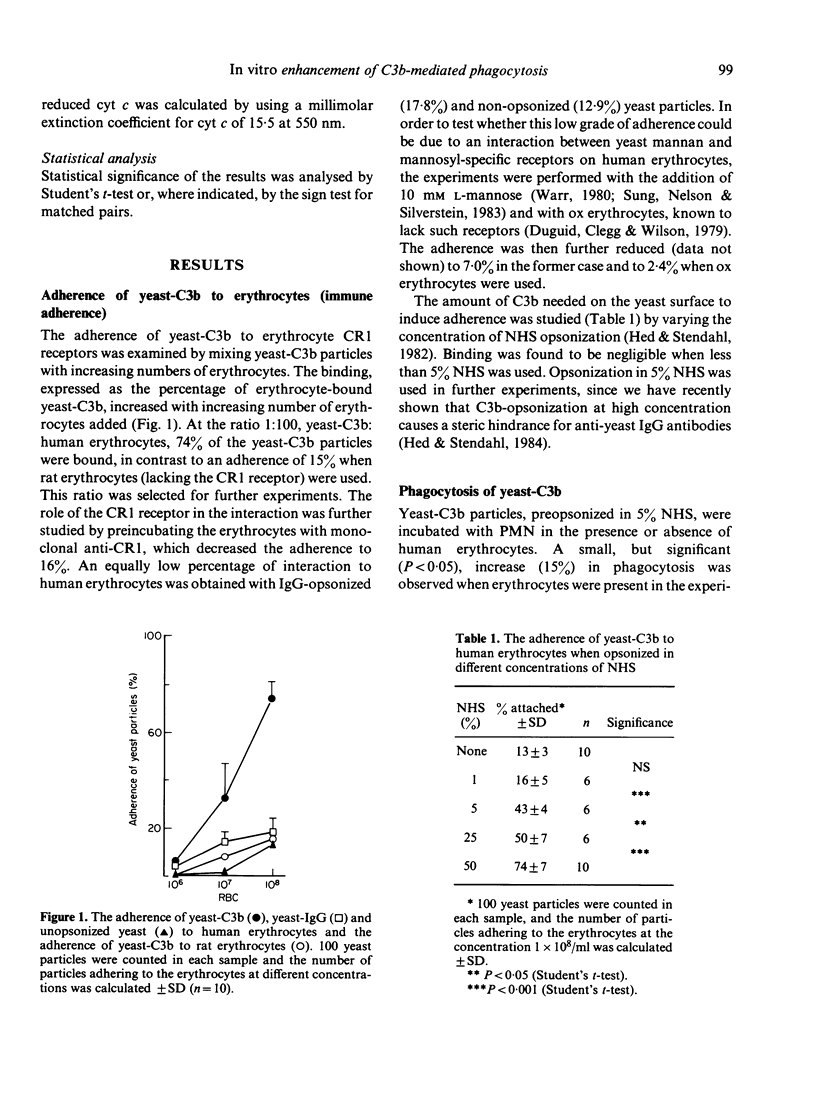
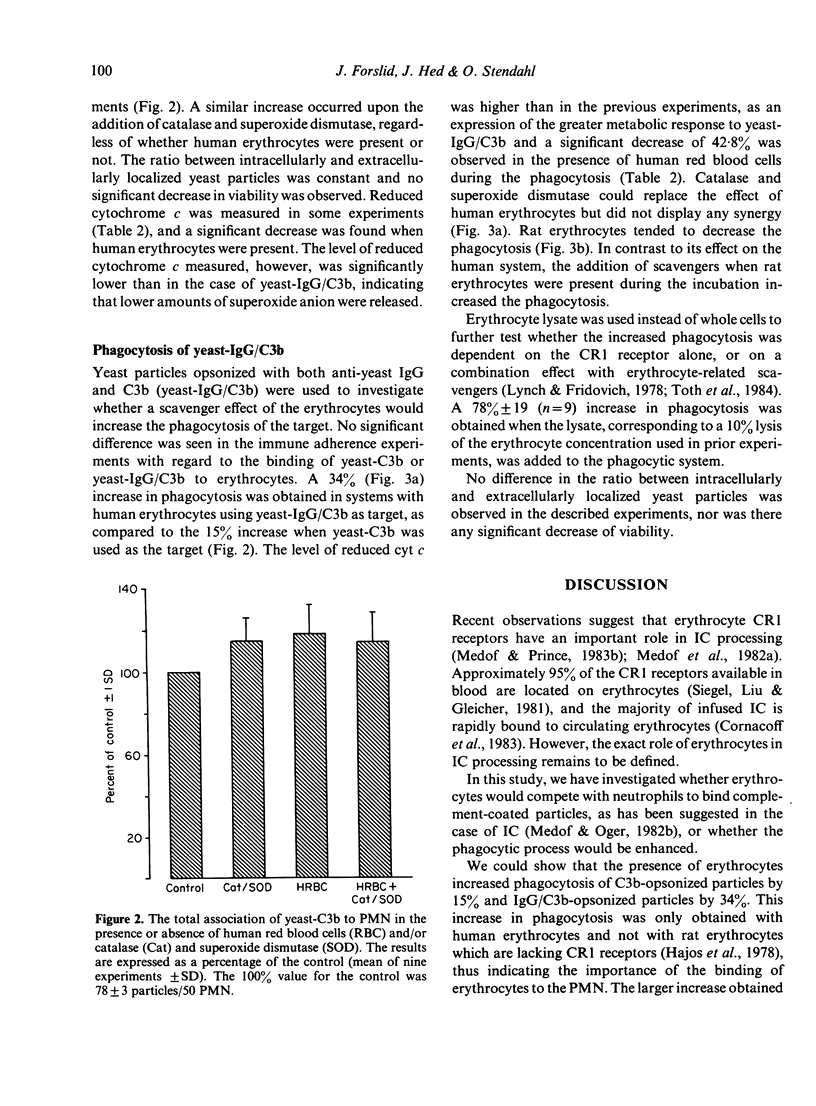
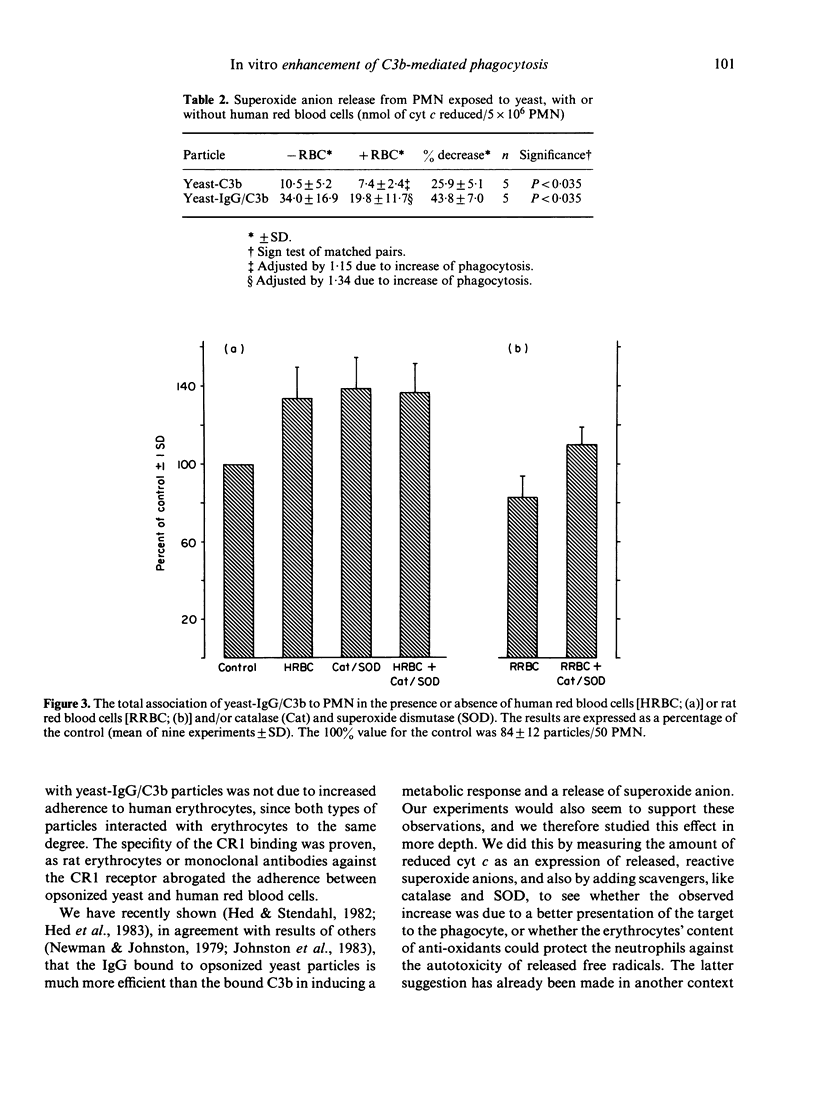
Human erythrocyte CR1 receptors have been shown to bind complement-fixing immune complexes and, thus, facilitate their elimination from the circulation. The autotoxic effect of free radicals released from phagocytes during phagocytosis can be alleviated by scavengers like catalase and superoxide dismutase. Erythrocytes are known to contain these antioxidants. This study showed that 74% of opsonized yeast particles adhered to human erythrocytes. No difference was seen between yeast opsonized with C3b and yeast opsonized with both IgG and C3b. This adherence was due to the C3b receptor (CR1), as monoclonal antibodies against the CR1 receptor could abrogate the adherence. The yeast phagocytosis by neutrophils was increased by 15% when yeast-C3b was used, and by 34% when yeast-IgG/C3b was used in the presence of human red blood cells. The increase of phagocytosis was not seen when rat erythrocytes (lacking CR1) were present. The cytochrome c reduction decreased with the presence of human erythrocytes during phagocytosis, indicating a scavenging effect on the superoxide anions. The addition of scavengers or erythrocyte lysate, instead of erythrocytes, enhanced phagocytosis of yeast-IgG/C3b to at least the same extent as the erythrocytes. These observations suggest that human erythrocytes primarily enhance phagocytosis through the scavenging effect of those erythrocytes which are concurrently attached with the prey through its CR1 receptor, and then attached to the PMN.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornacoff J. B., Hebert L. A., Smead W. L., VanAman M. E., Birmingham D. J., Waxman F. J. Primate erythrocyte-immune complex-clearing mechanism. J Clin Invest. 1983 Feb;71(2):236–247. doi: 10.1172/JCI110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Naiem M., Mason D. Y., Stein H. Human complement (C3b) receptors defined by a mouse monoclonal antibody. Immunology. 1982 Apr;45(4):645–653. [PMC free article] [PubMed] [Google Scholar]
- Hajos S. E., Margni R. A., Perdigón G., Manghi M., Olivera R. Binding of immunoglobulins and immune complexes to erythrocytes of vertebrates. Immunochemistry. 1978 Sep;15(9):623–628. doi: 10.1016/0161-5890(78)90034-2. [DOI] [PubMed] [Google Scholar]
- Hand W. L. Inhibition of cell-free oxidative bactericidal activity by erythrocytes and hemoglobin. Infect Immun. 1984 May;44(2):465–468. doi: 10.1128/iai.44.2.465-468.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Effect of erythrocyte ingestion on macrophage antibacterial function. Infect Immun. 1983 Jun;40(3):917–923. doi: 10.1128/iai.40.3.917-923.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hed J., Stendahl O. Differences in the ingestion mechanisms of IgG and C3b particles in phagocytosis by neutrophils. Immunology. 1982 Apr;45(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Hed J., Stendahl O., Sundqvist T. Differences in the association between the oxidase-dependent activity and plasma membrane receptors for IgG, C3b and concanavalin A of human neutrophils. FEBS Lett. 1983 Feb 21;152(2):212–216. doi: 10.1016/0014-5793(83)80382-2. [DOI] [PubMed] [Google Scholar]
- Iida K., Nussenzweig V. Complement receptor is an inhibitor of the complement cascade. J Exp Med. 1981 May 1;153(5):1138–1150. doi: 10.1084/jem.153.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem. 1977 Oct 10;252(19):6721–6728. [PubMed] [Google Scholar]
- Kärre K., Seeley J. K. Cytotoxic Thy 1,2-positive blasts with NK-like target selectivity in murine mixed lymphocyte cultures. J Immunol. 1979 Oct;123(4):1511–1518. [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978 Jul 10;253(13):4697–4699. [PubMed] [Google Scholar]
- Medof M. E., Iida K., Mold C., Nussenzweig V. Unique role of the complement receptor CR1 in the degradation of C3b associated with immune complexes. J Exp Med. 1982 Dec 1;156(6):1739–1754. doi: 10.1084/jem.156.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Lam T., Prince G. M., Mold C. Requirement for human red blood cells in inactivation of C3b in immune complexes and enhancement of binding to spleen cells. J Immunol. 1983 Mar;130(3):1336–1340. [PubMed] [Google Scholar]
- Medof M. E., Oger J. J. Competition for immune complexes by red cells in human blood. J Clin Lab Immunol. 1982 Jan;7(1):7–13. [PubMed] [Google Scholar]
- Roos D., Weening R. S., Wyss S. R., Aebi H. E. Protection of human neutrophils by endogenous catalase: studies with cells from catalase-deficient individuals. J Clin Invest. 1980 Jun;65(6):1515–1522. doi: 10.1172/JCI109817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel I., Liu T. L., Gleicher N. The red-cell immune system. Lancet. 1981 Sep 12;2(8246):556–559. doi: 10.1016/s0140-6736(81)90941-7. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Coble B. I., Dahlgren C., Hed J., Molin L. Myeloperoxidase modulates the phagocytic activity of polymorphonuclear neutrophil leukocytes. Studies with cells from a myeloperoxidase-deficient patient. J Clin Invest. 1984 Feb;73(2):366–373. doi: 10.1172/JCI111221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. S., Nelson R. S., Silverstein S. C. Yeast mannans inhibit binding and phagocytosis of zymosan by mouse peritoneal macrophages. J Cell Biol. 1983 Jan;96(1):160–166. doi: 10.1083/jcb.96.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K. M., Clifford D. P., Berger E. M., White C. W., Repine J. E. Intact human erythrocytes prevent hydrogen peroxide-mediated damage to isolated perfused rat lungs and cultured bovine pulmonary artery endothelial cells. J Clin Invest. 1984 Jul;74(1):292–295. doi: 10.1172/JCI111414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr G. A. A macrophage receptor for (mannose/glucosamine)-glycoproteins of potential importance in phagocytic activity. Biochem Biophys Res Commun. 1980 Apr 14;93(3):737–745. doi: 10.1016/0006-291x(80)91139-0. [DOI] [PubMed] [Google Scholar]



