Abstract
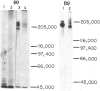
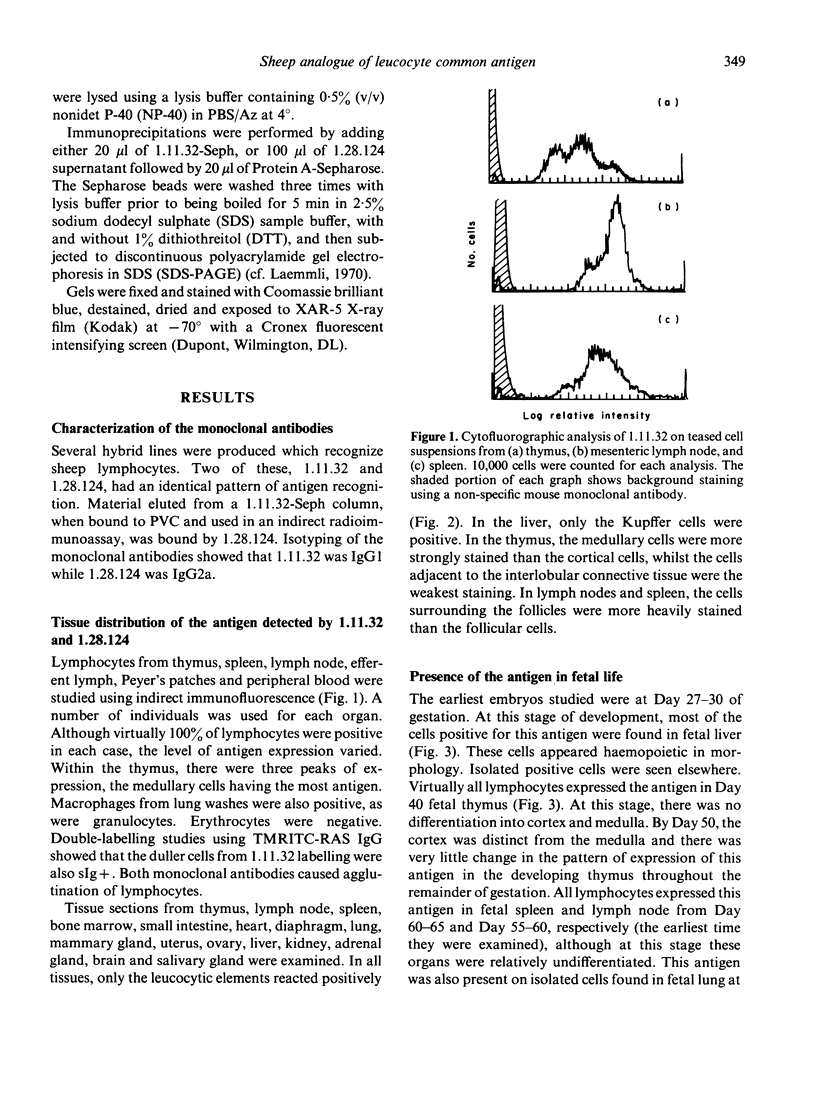
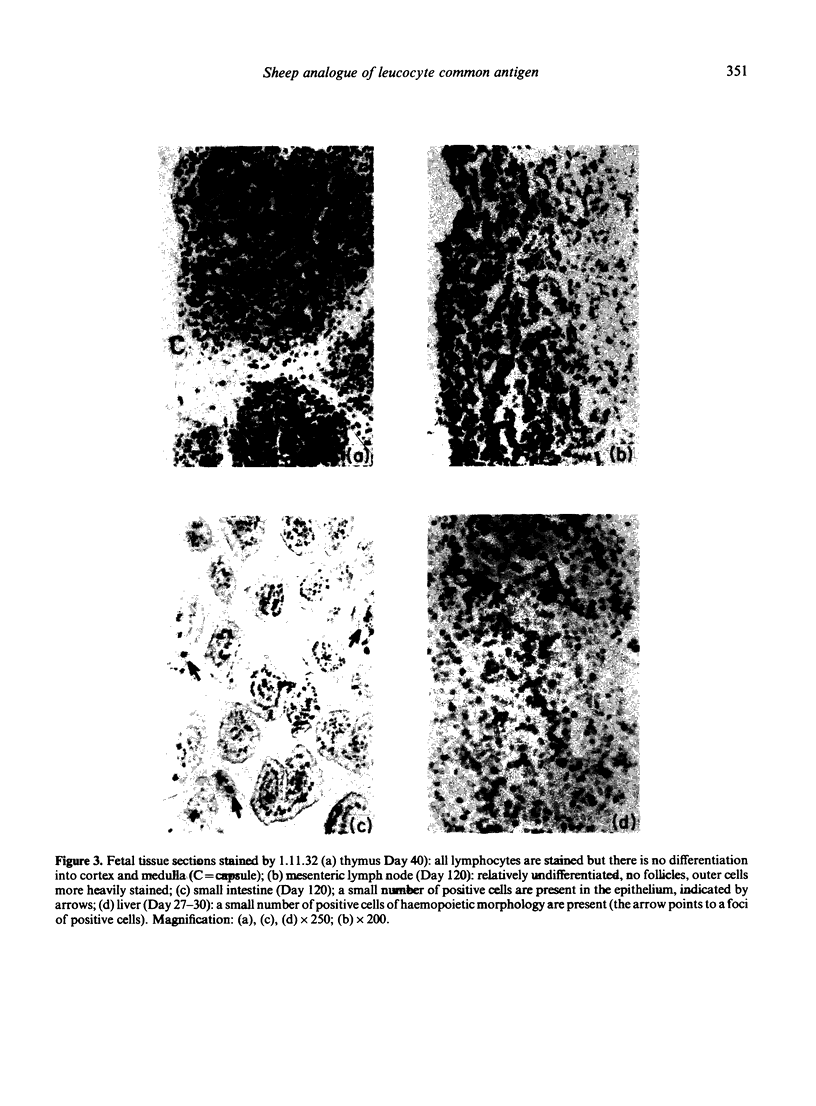
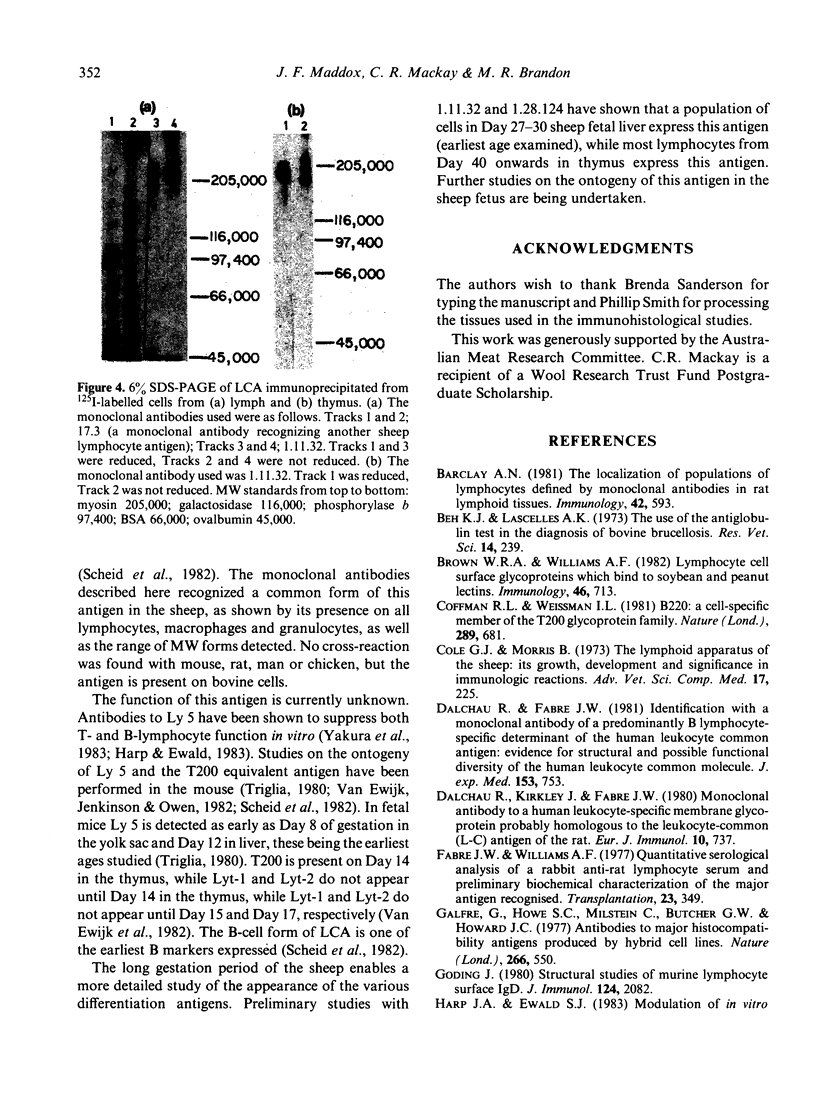
The tissue distribution and immunochemical properties of antigens recognized by two monoclonal antibodies 1.11.32 and 1.28.124 define the sheep analogue of the leucocyte common antigen found in rat, man and mouse. Histological and immunofluorescent studies show that this antigen is found on all lymphocytes, as well as other leucocytes but is absent from non-leucocytic cells. Immunochemical data show that a series of proteins of high molecular weight (190,000-225,000) are recognized, and histological studies show the presence of this antigen on a subpopulation of fetal liver cells as early as Day 27-30 of gestation, and on all fetal thymocytes from Day 40 of gestation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N. The localization of populations of lymphocytes defined by monoclonal antibodies in rat lymphoid tissues. Immunology. 1981 Apr;42(4):593–600. [PMC free article] [PubMed] [Google Scholar]
- Beh K. J., Lascelles A. K. The use of the antiglobulin test in the diagnosis of bovine brucellosis. Res Vet Sci. 1973 Mar;14(2):239–244. [PubMed] [Google Scholar]
- Brown W. R., Williams A. F. Lymphocyte cell surface glycoproteins which bind to soybean and peanut lectins. Immunology. 1982 Aug;46(4):713–726. [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Cole G. J., Morris B. The lymphoid apparatus of the sheep: its growth, development and significance in immunologic reactions. Adv Vet Sci Comp Med. 1973;17:225–263. [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification with a monoclonal antibody of a predominantly B lymphocyte-specific determinant of the human leukocyte common antigen. Evidence for structural and possible functional diversity of the human leukocyte common molecule. J Exp Med. 1981 Apr 1;153(4):753–765. doi: 10.1084/jem.153.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- Fabre J. W., Williams A. F. Quantitative serological analysis of a rabbit anti-rat lymphocyte serum and preliminary biochemical characterisation of the major antigen recognised. Transplantation. 1977 Apr;23(4):349–359. doi: 10.1097/00007890-197704000-00009. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Structural studies of murine lymphocyte surface IgD. J Immunol. 1980 May;124(5):2082–2088. [PubMed] [Google Scholar]
- Harp J. A., Ewald S. J. Modulation of in vitro immune responses by monoclonal antibody to T200 antigen. Cell Immunol. 1983 Oct 1;81(1):71–80. doi: 10.1016/0008-8749(83)90212-5. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Kon S., Takei T., Fujimoto J., Kikuchi K. Four distinct antigen systems on human thymus and T cells defined by monoclonal antibodies: immunohistological and immunochemical studies. Clin Exp Immunol. 1983 Jul;53(1):31–40. [PMC free article] [PubMed] [Google Scholar]
- Jordan R. K. Development of sheep thymus in relation to in utero thymectomy experiments. Eur J Immunol. 1976 Oct;6(10):693–698. doi: 10.1002/eji.1830061007. [DOI] [PubMed] [Google Scholar]
- LASCELLES A. K., MORRIS B. Surgical techniques for the collection of lymph from unanaesthetized sheep. Q J Exp Physiol Cogn Med Sci. 1961 Jul;46:199–205. doi: 10.1113/expphysiol.1961.sp001536. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morris R. J., Barber P. C. Fixation of Thy-1 in nervous tissue for immunohistochemistry: a quantitative assessment of the effect of different fixation conditions upon retention of antigenicity and the cross-linking of Thy-1. J Histochem Cytochem. 1983 Feb;31(2):263–274. doi: 10.1177/31.2.6131917. [DOI] [PubMed] [Google Scholar]
- Morris R. J., Williams A. F. Antigens on mouse and rat lymphocytes recognized by rabbit antiserum against rat brain: the quantitative analysis of a xenogeneic antiserum. Eur J Immunol. 1975 Apr;5(4):274–281. doi: 10.1002/eji.1830050412. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Loken M. R., Trowbridge I. S., Coffman R. L., Fitch F. W. High molecular weight lymphocyte surface proteins are structurally related and are expressed on different cell populations at different times during lymphocyte maturation and differentiation. J Immunol. 1982 Apr;128(4):1676–1684. [PubMed] [Google Scholar]
- Scheid M. P., Landreth K. S., Tung J. S., Kincade P. W. Preferential but nonexclusive expression of macromolecular antigens on B-lineage cells. Immunol Rev. 1982;69:141–159. doi: 10.1111/j.1600-065x.1983.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Spickett G. P., Brandon M. R., Mason D. W., Williams A. F., Woollett G. R. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J Exp Med. 1983 Sep 1;158(3):795–810. doi: 10.1084/jem.158.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker J. W., Heusser C. H. Methods for binding cells to plastic: application to a solid-phase radioimmunoassay for cell-surface antigens. J Immunol Methods. 1979;26(1):87–95. doi: 10.1016/0022-1759(79)90044-9. [DOI] [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Triglia D. Expression of Ly-5 on yolk sac and fetal liver cells of the mouse. Immunogenetics. 1980;11(3):303–307. doi: 10.1007/BF01567796. [DOI] [PubMed] [Google Scholar]
- Tung J. S., Scheid M. P., Palladino M. A. Different forms of Ly-5 within the T-cell lineage. Immunogenetics. 1983;17(6):649–654. doi: 10.1007/BF00366132. [DOI] [PubMed] [Google Scholar]
- Van Ewijk W., Jenkinson E. J., Owen J. J. Detection of Thy-1, T-200, Lyt-1 and Lyt-2-bearing cells in the developing lymphoid organs of the mouse embryo in vivo and in vitro. Eur J Immunol. 1982 Apr;12(4):262–271. doi: 10.1002/eji.1830120403. [DOI] [PubMed] [Google Scholar]
- Yakura H., Shen F. W., Bourcet E., Boyse E. A. On the function of Ly-5 in the regulation of antigen-driven B cell differentiation. Comparison and contrast with Lyb-2. J Exp Med. 1983 Apr 1;157(4):1077–1088. doi: 10.1084/jem.157.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]