Abstract
The antimycobacterial role of eosinophil peroxidase (EPO), one of the most abundant granule proteins in human eosinophils, was investigated. Our data indicate that purified EPO shows significant inhibitory activity towards Mycobacterium tuberculosis H37Rv. On a molar basis, this activity was similar to that exhibited by neutrophil myeloperoxidase (MPO) and was both dose and time dependent. In contrast to the activity of MPO, which requires H2O2, EPO also exhibited anti-M. tuberculosis activity in the absence of exogenously added peroxide. Morphological evidence confirmed that the mechanism of action of EPO against mycobacteria differs from that of MPO. While MPO kills M. tuberculosis H37Rv exclusively in the presence of hydrogen peroxide, it does not induce morphological changes in the pathogen. In contrast, EPO-treated bacteria frequently had cell wall lesions and eventually underwent lysis, either in the presence or in the absence of H2O2.
Granulocytes respond at the onset of microbial invasion and migrate from the blood into tissues, where they participate in the early inflammatory response. However, the involvement of these cells in the host response against Mycobacterium tuberculosis is controversial (9, 16, 17, 39, 40). Mycobacterium species, including M. tuberculosis, induce neutrophil influx in animal models (3, 4, 5, 6, 45). Isolated neutrophils ingest M. tuberculosis and initiate a respiratory burst (31) that is capable of killing M. tuberculosis (9). In addition, neutropenia has been cited as a risk factor for mycobacterial infection (32). It has been shown that eosinophilic granulocytes may also participate in the inflammatory response initiated by M. tuberculosis (12), but even in this case the role of these granulocytes remains to be established (27). In contrast, convincing evidence for the role of macrophages in both intracellular replication of and resistance to M. tuberculosis has been obtained (26). These findings have led researchers to focus their efforts on the M. tuberculosis-inactivating mechanisms of macrophages instead of granulocytes. In the latter cells, however, at least two kinds of antimycobacterial proteins have been described: peroxidases and defensins (8, 29, 38).
Leukocyte peroxidases are known to have bactericidal activity against a wide spectrum of microorganisms (18, 29). Recently, we reported that neutrophil myeloperoxidase (MPO) purified from human granulocytes is able to kill both M. tuberculosis H37Rv and clinical isolates (8). This activity could be relevant in vivo since neutrophils precede activated macrophages at an M. tuberculosis-infected site. However, even in neutrophils, M. tuberculosis can hinder degranulation and reduce contact between the intraphagosomal released peroxidase and the pathogen (37). Therefore, MPO can interact with the M. tuberculosis surface only in the extracellular medium or after endocytosis, since fusion of endocytic vesicles with the phagosome is not eliminated in M. tuberculosis-infected cells (13, 42). The latter conditions, however, are unlikely to allow optimal activity of MPO in vivo, since in the extracellular environment the concentration of H2O2, the peroxidase cosubstrate, is low due to both intra- and extracellular degradation. Macrophages also endocytose MPO to a small extent (54, 55). Therefore, we evaluated the possibility that eosinophil peroxidase (EPO) can have antimycobacterial effects, since this enzyme is present at high levels in the specific granules of eosinophils (1, 52) and is endocytosed to a large extent by phagocytes (54, 55). Accordingly, it should have the ability to reach the M. tuberculosis-containing phagosome interior, as reported for other membrane-borne vesicles (13, 42). Furthermore, EPO is known to exhibit activity at H2O2 concentrations lower than that required by MPO (33). Finally, EPO has been reported to have parasitocytocidal or cytocidal effects, even in the absence of the cosubstrate (23, 36).
In this study, we showed that EPO is able to kill M. tuberculosis H37Rv at concentrations similar to those required for MPO killing of M. tuberculosis. However, this antimycobacterial activity does not require hydrogen peroxide. In the presence of the cosubstrate, no viable M. tuberculosis cells were found after 24 h of exposure to EPO. Morphological evidence also demonstrated that the damage induced by EPO on M. tuberculosis is different from that induced by MPO. The possibility that EPO could be exploited as an anti-M. tuberculosis agent in an animal model is discussed below.
MATERIALS AND METHODS
Reagents.
Phosphate-buffered saline (PBS), hydrogen peroxide, protamine, polylysine guaiacol, sodium chloride, sodium iodide, sodium bromide, aminotriazole, 3,3′,5,5′-tetramethylbenzidine (TMB), and cetyltrimethylammonium bromide were purchased from Sigma Chemical Co., St. Louis, Mo. All other chemicals were reagent grade or higher.
Peroxidases.
MPO was obtained as described previously (54) from human blood granulocytes. Purified MPO had a ratio of absorption in the Soret region at 428 nm to absorption at 280 nm (Rz) (commonly used as a criterion of purity for heme peroxidases) of 0.75, which indicates a high level of purity (51); its concentration in 25 mM phosphate buffer (pH 7.0) was 1.6 mg/ml (10.7 μM).
Highly purified EPO was obtained from eosinophilic human blood. Leukocytes, prepared as previously described (53), were extracted at 0°C for 12 h with 0.1 M sodium acetate buffer (pH 4.7) containing 0.1 M sodium sulfate and 0.075% cetyltrimethylammonium bromide. The procedure was repeated twice, and after 45 min of centrifugation at 20,000 × g, the two supernatants were combined (final volume, 25 ml). The eosinophil extract, extensively dialyzed against 25 mM phosphate buffer (pH 7.0) containing 0.5 M NaCl, was centrifuged again for 45 min at 20,000 × g and fractionated in two steps by ion-exchange chromatography by using a fast protein liquid chromatography system (Pharmacia, Uppsala, Sweden). In the first step, the anionic components were removed from the extract by passage through a Mono-Q column, which retained anions and excluded cations. The unbound proteins, which included EPO, were subsequently passed over a Mono-S column (strong cationic exchanges). Fractions (0.5 ml) were collected after a linear gradient of 0 to 2 M NaCl dissolved in 25 mM phosphate buffer (pH 7.4) was applied. EPO eluted from the column at 1.7 to 2.0 M NaCl. The purified enzyme had the typical absorbance spectrum reported previously (11, 34) and had an Rz of 1.10, which is almost the highest purification index reported to date (11, 34). The purity of the enzyme was also demonstrated by the results of nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which produced a single band at approximately 66 kDa, as expected (see below). The enzymatic activity of EPO (protein concentration, 0.32 mg/ml [5 μM] in 25 mM phosphate buffer [pH 7.0]) was analyzed by using either the guaiacol oxidation method (41) or the TMB method (33).
Where indicated below, we exposed bacteria to EPO that were treated with the peroxidase inhibitor aminotriazole in order to evaluate the dependence of the EPO antimycobacterial effect on the enzymatic activity. EPO from the stock solution (5 μM) was exposed to 5 mM aminotriazole and 100 nM H2O2. After 20 min, the mixture was extensively dialyzed against 25 mM phosphate buffer (pH 7.0) and added to bacteria at a final concentration of 1 μM. The enzymatic activity of aminotriazole-treated EPO was strongly (85%) inhibited, as judged by the guaiacol oxidation method.
Bacteria and bactericidal activity.
In order to evaluate the antitubercular effect of EPO, we utilized M. tuberculosis strain H37Rv (Pasteur Institute, Paris, France). The bacteria were grown on Lowenstein-Jensen medium for 3 to 4 weeks, resuspended at a concentration of 6 × 106 to 8 × 106 cells/ml in Middlebrook 7H9 broth (Difco Laboratories, Detroit, Mich.) without supplements, and incubated for different lengths of time (0 to 24 h) in PBS either in the presence or in the absence of 0.25 mM hydrogen peroxide (depending on the enzymatic analysis of EPO shown in Fig. 1) along with different amounts of purified EPO or MPO. Each mixture was appropriately diluted in PBS, and each dilution was plated in triplicate on Middlebrook 7H11 agar (Difco) enriched with OADC supplement (Difco). The agar plates were then incubated at 37°C, and the numbers of CFU were determined after 3 to 4 weeks. It was interesting that EPO (or MPO) treatment did not increase low-grade spontaneous bacterial clumping either in the presence or in the absence of H2O2, as judged by light microscope observation after 2% glutaraldehyde fixation. An increase in clumping was observed by scanning microscope analysis (see below). This effect seemed to be induced by the procedure used to prepare bacteria for the ultrastructural analysis, since it was not observed with the light microscope. When the bacteria were almost completely lysed, some clumps were observed. In our opinion this did not lead to overestimation of the killing effect, since the clumps were composed primarily of bacterial fragments and contained few, if any, entire bacteria. When present, whole bacteria did not appear to exhibit increased aggregation.
FIG. 1.
Absorbance spectrum of pure EPO. An Rz of 1.10 was calculated (see Materials and Methods). The inset shows the electrophoretic purity of the same batch of EPO, as determined by nonreducing SDS-PAGE. Left lane, 3 μg; right lane, 6 μg. The gel was stained with Coomassie blue.
The halide dependence of the antimycobacterial activity of EPO was tested by the method of Klebanoff et al. (28). Briefly, EPO (final concentration, 0.25 μM, corresponding to about 100 to 150 pmol/106 M. tuberculosis cells) was added to a suspension containing 2 × 105 H37Rv cells in 25 mM sodium phosphate buffer (pH 7.0) supplemented with 0.067 M sodium sulfate and, where indicated, 0.1 M NaCl, 0.2 mM NaI, or 0.2 mM NaBr either in the presence or in the absence of 0.25 mM H2O2. After 60 min of incubation at 37°C, the suspensions were appropriately diluted, and the CFU were counted as described above.
SEM.
The effects of MPO and EPO on the morphology of M. tuberculosis were examined by scanning electron microscopy (SEM). Suspensions containing 1 × 106 to 5 × 106 M. tuberculosis cells/ml were incubated for different lengths of time (0, 45, and 120 min) in PBS either in the presence or in the absence of H2O2 (final concentration, 0.25 mM) along with different amounts of EPO or MPO or 100 μg of protamine per ml. After each time interval, portions were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) for 90 min. The bacteria were washed twice in PBS, placed on glass coverslips (previously coated with poly-l-lysine) for 1 h, and then processed for SEM. Briefly, the coverslips were dehydrated in a graded 50 to 100% ethanol series, dried in a CO2 apparatus (Bal-Tec; EM Technology and Application, Furstentum, Liechtenstein), sputter coated with gold in a Edwards S150A apparatus (Edwards High Vacuum, Crawley, West Sussex, United Kingdom), and examined with a Leica Stereoscan 430i scanning electron microscope (Leica Cambridge Ltd., Cambridge, United Kingdom).
Transmission electron microscopy (TEM).
Suspensions of M. tuberculosis, which were either not treated or exposed for 2 h to either EPO alone (0.2 μM) or EPO (0.2 μM) and H2O2 (0.25 mM) in the presence of Cl− (present in PBS), were fixed for 1 h in a solution of 2% glutaraldehyde (Serva, Heidelberg, Germany) in 0.1 M cacodylate buffer (pH 7.3) containing 0.03 M CaCl2. The fixed bacteria were washed twice (10 min each) with 0.1 M cacodylate buffer and then postfixed with 1% osmium tetroxide for 1 h at 4°C. Postfixed bacteria were dehydrated with an ascending ethanol series ending with 100% ethanol and then embedded in Dow epoxy resin (DER332; Unione Chimica Europea, Milan, Italy) and DER732 (Serva), as previously described by Zabucchi et al. (30, 54). Ultrathin sections were prepared with an Ultrathome III (LKB, Pharmacia) and then were double stained with lead citrate and uranyl acetate and observed with a transmission electron microscope (EM208; Philips, Eindhoven, The Netherlands).
RESULTS
EPO isolated from eosinophilic blood was first tested for purity and characterized enzymatically. In nonreducing SDS-PAGE (Fig. 1, inset), the enzyme produced a single band when 3 to 6 μg of protein was loaded. The absorbance spectrum is shown in Fig. 1 and has an absorption maximum near 415 nm that is typical of EPO (11, 34). Its Rz (the Rz is commonly used as a criterion of purity for heme peroxidases) was 1.10, suggesting that the level of purity was very high (11).
Figure 2 shows the H2O2 and halogen dependence of EPO oxidative activity at pH 5.5 (with TMB as the substrate) or at pH 7.0 (with guaiacol as the substrate). At pH 5.5, optimal activity was obtained in the presence of 0.4 mM H2O2 and bromide. In the presence of chloride, the maximal activity was similar, but the optimal concentration of hydrogen peroxide was lower (0.25 mM). In the absence of halogen or in the presence of iodide, the optimal activity was less than one-half that observed with chloride or bromide and was observed at H2O2 concentrations higher than 0.4 mM. The opposite pattern was observed at pH 7.0. In this case, the maximal activity was observed in the absence of halogen or in the presence of iodide and at a lower concentration of H2O2 (0.25 mM). In the presence of chloride or bromide, the EPO activity was about 35% lower than the activity observed with iodide and did not vary significantly in the hydrogen peroxide concentration range from 0.25 to 0.6 mM. No activity was found when the EPO enzymatic activity was tested in the absence of H2O2 either at pH 5.5 or at pH 7.0.
FIG. 2.
Enzymatic characterization of pure EPO. The values are the means of three determinations. (a) H2O2 dose response and halide dependence of the TMB-oxidizing activity of EPO. The ordinate indicates the change in the optical density (OD) per minute obtained with 5 pmol (contained in 1 μl of pure EPO) in the assay mixture (final volume, 1 ml) and recalculated (and expressed [103]) as obtained with 1 ml of EPO (original batch concentration) in the same assay. (b) H2O2 dose response and halide dependence of the guaiacol-oxidizing activity of EPO. The ordinate indicates the change in the optical density per minute obtained with 5 pmol (contained in 1 μl of pure EPO) in the assay mixture (final volume, 1 ml) and referred to 1 ml of EPO (original batch concentration).
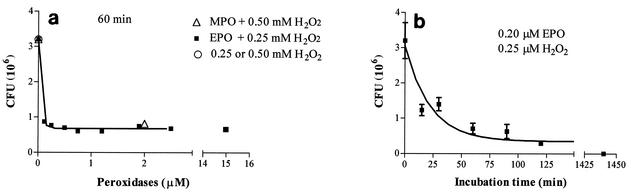
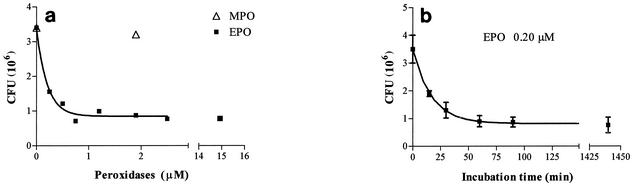
Figure 3a shows the dose response of the bactericidal activity of EPO against M. tuberculosis H37Rv in the presence of H2O2 (0.25 mM) and chloride (100 mM) after 1 h of coincubation. The maximum effect was observed with 0.25 to 0.5 μM EPO, since after the same incubation time with 15 μM EPO, the anti-M. tuberculosis effect was not greater. MPO has the same effect at a concentration of 2 μM. H2O2 alone has no bactericidal activity. Figure 3b shows the time course of the antimycobacterial effect of EPO. The maximum effect, as monitored after short incubation times, was observed after about 60 min, when the number of CFU was reduced to about 22% of their initial number. However, when the incubation time was increased to 24 h, no CFU were found, demonstrating that under these conditions EPO completely killed the pathogen. When the antimycobacterial effect of EPO was assayed in the absence of hydrogen peroxide, it was found that EPO had bactericidal activity. Figure 4 shows that this activity, whose maximum value was observed when the concentration of EPO was 0.5 to 0.7 μM, was comparable to the activity exhibited by the enzyme in the presence of H2O2, even though in the former case the antibacterial activity was slightly weaker. Under these conditions, increasing the incubation time to 24 h did not result in a stronger effect. Conversely, in the absence of H2O2, 2 μM MPO had no mycobactericidal activity at all. Increasing the MPO concentration to 10 μM and increasing the incubation time to 24 h did not improve the anti-M. tuberculosis activity of MPO in the absence of hydrogen peroxide (data not shown).
FIG. 3.
Dose-response curve for the inhibitory effect of EPO and H2O2 on the growth of M. tuberculosis H37Rv. The values are the means ± standard deviations of at least three experiments or the means of two experiments. (a) Symbols: ▪, number of CFU after 60 min of incubation at 37°C in the presence of EPO and H2O2 (final concentration, 0.25 mM); ▵, number of CFU after 60 min of incubation at 37°C in the presence of MPO and H2O2 (final concentration, 0.5 mM); ○, number of CFU after 60 min of incubation at 37°C in the presence of H2O2 alone. (b) Time course of the inhibitory effect of 0.2 μM EPO and H2O2 on the growth of M. tuberculosis H37Rv.
FIG. 4.
Dose-response curve for the inhibitory effect of EPO on the growth of M. tuberculosis H37Rv. The values are the means ± standard deviations of at least three experiments or the means of two experiments. (a) Symbols: ▪, number of CFU after 60 min of incubation at 37°C in the presence of EPO alone; ▵, number of CFU after 60 min of incubation at 37°C in the presence of MPO. (b) Time course of the inhibitory effect of 0.2 μM EPO on the growth of M. tuberculosis H37Rv.
The findings shown in Fig. 2 suggest that the enzymatic activity of EPO is dependent on the presence of specific halides. These findings confirm those reported by other authors (15, 46, 50) showing that EPO is more active with iodide or bromide, even though it has been reported that the enzyme works with chloride as well (10, 24, 25, 28). Accordingly, we evaluated the halide dependence of the antimycobacterial activity of EPO. To do this, we exposed M. tuberculosis to EPO and different halides for 2 h either in the presence or in the absence of H2O2.
Figure 5b shows that even in the absence of halides, EPO plus H2O2 exhibited killing activity against M. tuberculosis, which almost reached the maximum level of activity in the presence of halides. However, changing the halide did not significantly influence the anti-M. tuberculosis activity. In the absence of H2O2 (Fig. 5a), the killing activity was lower, but similarly, varying the halide had no effect.
FIG. 5.
Anti-M. tuberculosis activity of EPO: halide dependence and requirement for enzymatic activity. A suspension containing 2 × 105 H37Rv cells in 25 mM sodium phosphate buffer (pH 7.0) supplemented with 0.067 M sodium sulfate and (as indicated) 0.1 M NaCl, 0.2 mM NaI, or NaBr either in the presence or in the absence of 0.25 mM H2O2 was exposed to 0.2 μM EPO for 60 min of incubation at 37°C. See Materials and Methods for details. Where indicated, aminotriazole (AMT)-treated EPO was used.
As expected, inhibition (85%) of the enzymatic activity of EPO with aminotriazole did not influence the killing activity exhibited by EPO alone, while only slight inhibition was observed in the presence of hydrogen peroxide.
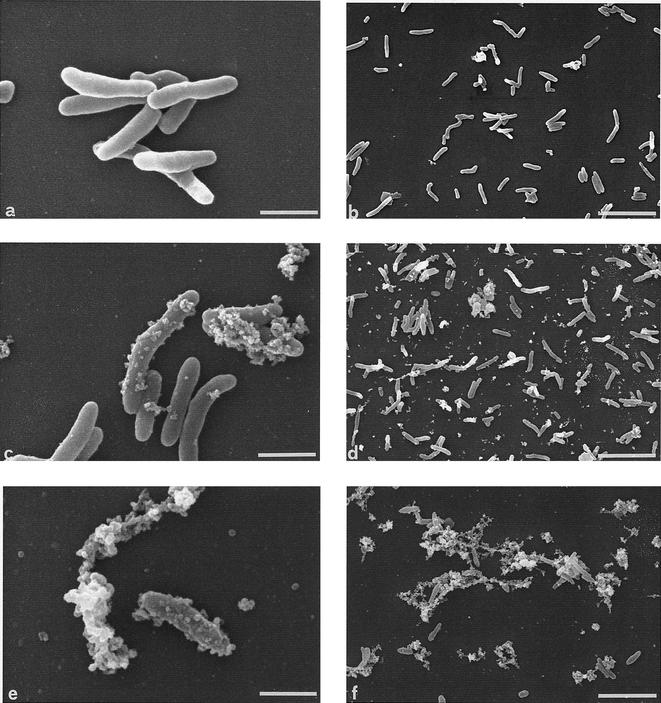
Figure 6 shows the morphology of peroxidase-treated M. tuberculosis as observed by SEM. Three types of morphology (Fig. 6a, c, and e) were observed; control and MPO-treated bacteria had the morphology shown in Fig. 6a, and the morphology shown in Fig. 6c was observed mainly with bacteria that were treated for 45 min with EPO, either in the presence or in the absence of H2O2. The surfaces of these microorganisms appeared to be covered by structures that were absent from control M. tuberculosis cells. These structures could be either blebs from the bacterial body or bacterial fragments derived from lysed M. tuberculosis cells and attached to intact cells.
FIG. 6.
SEM of EPO-treated M. tuberculosis H37Rv. (a, c, and e) High magnifications of the three main types of morphological changes observed. Bars = 1 μm. (b, d, and f) Low magnifications of the time course of the morphological changes induced by EPO (0.2 μM) and H2O2 (0.25 mM). (b) M. tuberculosis at the beginning of the incubation (time zero); (d) M. tuberculosis after 45 min of incubation with EPO and H2O2; (f) M. tuberculosis after 2 h of incubation with EPO and H2O2. Bars = 5 μm.
Among the mycobacteria treated with EPO for 120 min in the presence or in the absence of H2O2, the morphology shown in Fig. 6e predominated. The structures appeared to be clumps of blebs or fragments, and many bacterium-derived blebs or fragments were present in the background.
Figures 6b, d, and f show the time course of the changes induced by EPO and H2O2 in M. tuberculosis at a low magnification. Figure 6b shows the appearance of bacteria at the time of EPO addition (time zero). After 45 min of incubation (Fig. 6d), most M. tuberculosis cells showed the “blebbing” morphology shown in Fig. 6c, while only some of the cells were lysed. After 120 min (Fig. 6f), most bacteria were completely lysed, and only some of them still had a rod-like appearance; in addition, many clusters of bacterium-derived blebs or fragments were present. In the absence of H2O2 EPO-treated bacteria showed (although to a lesser extent) the same morphological changes induced by EPO plus H2O2. Specifically, after 45 min of incubation many bacteria had the blebbing appearance, and severely damaged bacteria were rarely seen. After 120 min of incubation more than 50% the M. tuberculosis cells were lysed. These morphological alterations were dependent on the presence of EPO. They were completely absent in M. tuberculosis exposed to MPO, H2O2, or MPO and H2O2, even if under the latter conditions they were killed. Since EPO is more cationic than MPO (52), we investigated whether other highly cationic compounds are able to mimic the activity of EPO that induces cell wall alterations in M. tuberculosis. Incubating M. tuberculosis with protamine (100 μg/ml) did not induce any structural alterations or bacterial killing (data not shown), suggesting that it is not the positive charge of EPO, by itself, that affects the surface integrity of the pathogen.
Observation of EPO-treated M. tuberculosis by TEM confirmed the findings obtained with SEM. After 2 h of incubation with either EPO alone or EPO plus H2O2, M. tuberculosis cells were severely damaged (Fig. 7). When this method was used, blebs were frequently seen under the former conditions (Fig. 7b, inset), but they were not as abundant as would have been expected on the basis of the SEM analysis. Conversely, bacteria that were undergoing lysis (mainly with EPO alone) (Fig. 7b) or that were completely lysed (mainly with EPO plus H2O2) (Fig. 7c) were abundant.
FIG.7.
TEM of EPO-treated M. tuberculosis H37Rv. The incubation time was 120 min. The assay conditions were exactly the same as those described in the legend to Fig. 6. (a) Untreated M. tuberculosis. Magnification, ×26,000; bar, 0.5 μm. (b) M. tuberculosis treated with EPO alone. M. tuberculosis cells are damaged, but many of them are still intact. Magnification, ×26,000; bar, 0.5 μm. In the inset two M. tuberculosis cells with blebbing surfaces are shown (magnification, ×80,000; bar, 0.1 μm). (c) M. tuberculosis treated with EPO and H2O2. M. tuberculosis cells are severely damaged, and there are few intact cells. Bacterial fragments and debris are seen frequently. Magnification, ×26,000; bar, 0.5 μm. In the inset completely lysed M. tuberculosis cells are shown (magnification, ×40,000; bar, 0.5 μm).
DISCUSSION
In this report, we show that EPO exhibits bactericidal activity against M. tuberculosis. After demonstrating the high level of purity of our EPO preparation, we characterized its enzymatic activity at pH 5.5 and 7.0 in order to find the most appropriate assay conditions for evaluating its bactericidal activity. On the basis of our findings and considering that (i) the EPO activity in the presence of chloride is never markedly different from the maximal activity recorded in the presence of other halides, (ii) chloride is the main halogen found in biological systems, and (iii) the pH of the M. tuberculosis-containing phagosome falls in the range from 6.0 to 7.0 (21, 49), we chose to analyze the anti-M. tuberculosis activity of EPO plus chloride at pH 7.0 in the presence of 0.25 mM H2O2, which ensured high EPO activity at a wide range of pH values (pH 5.5 to 7.0). These conditions can be considered to be representative of the conditions for EPO activity in vivo, even though more favorable conditions for the enzymatic activity of EPO might be found in specific compartments.
When these assay conditions were used, EPO exhibited strong anti-M. tuberculosis activity. Varying the halide included in the assay mixture did not significantly improve the anti-M. tuberculosis activity of EPO, which maintained its killing activity even in the absence of a halide.
The enzyme exhibited consistent killing activity against the pathogen even when its cosubstrate, H2O2, was absent. The bactericidal activity of EPO in the absence of peroxide is not surprising, since a similar activity against both bacteria and parasites has been reported by other authors (23, 36). Our findings do not allow attribution of this activity to the high positive charge of EPO as reported for other eosinophilic cytotoxic proteins (7), since at high concentrations protamine did not mimic this effect. It is unclear how EPO can act in the absence of H2O2. One possibility is that even traces of H2O2 generated by M. tuberculosis escaping the intrabacterial dismutase activity of catalase might trigger the enzymatic activity of EPO. It is very likely that the M. tuberculosis killing activity of EPO could be greatly improved by its capacity to bind to anionic cell surfaces, where it can accumulate and trap even traces of peroxide which may come from either the M. tuberculosis interior in our in vitro model or from other bystander cells in vivo. Another possibility is that EPO can produce H2O2 by itself. However, this possibility seems unlikely since the activity of peroxidases proceeds in the presence of NADH (or NADPH) as a substrate (52), which in intact bacterial cells is separated from EPO by both the cell wall and the plasma membrane and, therefore, cannot be oxidized to produce H2O2. The observation that aminotriazole, a strong peroxidase inhibitor (14), did not inhibit the antimycobacterial activity of EPO supports the concept that EPO also works independent of its enzymatic activity through an unknown mechanism. However, the inhibition of aminotriazole was not complete and reached only about 85%. Therefore, this finding cannot exclude the possibility that residual enzymatic activity can act on the pathogen.
We also examined the morphology of EPO-treated M. tuberculosis by both SEM and TEM. As determined by SEM analysis, M. tuberculosis preparations treated with EPO or EPO plus H2O2 frequently showed blebs and fragments, suggesting that there was increased cellular permeability. However, TEM analysis, besides confirming the severe damage to M. tuberculosis induced by EPO, revealed that the blebs were not abundant, as suggested by their appearance in SEM preparations. On this basis, we think that the structures covering EPO-treated M. tuberculosis cells are not blebs budding from the bacterial body but are fragments derived from bacteria undergoing lysis and attached to intact cells. Therefore, EPO activity causes strong alterations of the cell wall, fragmentation, and eventually, after 120 min of incubation, lysis of the major part of the pathogens. These changes were particularly noticeable when H2O2 was present. Among the proteins known to exhibit bactericidal activity against mycobacteria, EPO seems to be the most potent. The other known mycobactericidal proteins, such as defensins (38, 43, 44), protregrins (35), NK lysins (2), and granulysin (19, 48), exhibit significant mycobactericidal activity starting at a concentration of 10 μM. After 4 to 5 h, a minimal effect was found, and at least 24 h was required before significant activity of these proteins was observed. Additionally, glutathione and S-nitrosoglutathione are able to kill mycobacteria, but in this case the activity is even weaker, on a molar basis, than the activities of the proteins mentioned above. These tripeptides exhibit significant killing activity against Mycobacterium bovis at a concentration of 2.5 mM after a period of at least 40 h (20). Conversely, EPO is active at lower concentrations, with significant killing of M. tuberculosis observed at a concentration of 0.25 μM in the absence of H2O2. In the presence of H2O2, EPO shows activity at lower concentrations (0.12 μM), and the maximum effect is observed with 0.25 to 0.5 μM EPO. Furthermore, EPO exhibits its antimycobacterial effect in a shorter time; after 60 min of incubation about 80% of the bacteria are dead, and in the presence of H2O2, no CFU are observed after 24 h. Moreover, EPO is the only antimycobacterial protein capable of inducing bacterial fragmentation and lysis, a process that is evident even after 45 min of treatment and ensures definitive bacterial inactivation.
Recently, we showed that MPO, the peroxidase in neutrophilic granulocytes, kills M. tuberculosis. However, the mechanism of the mycobactericidal activity of EPO differs from that of MPO. While MPO kills M. tuberculosis only in the presence of H2O2 and apparently does not induce alterations in pathogen morphology, EPO is active even in the absence of exogenously added hydrogen peroxide and strongly perturbs the cell wall of M. tuberculosis, with eventual lysis of the microorganism.
In the presence of exogenously added H2O2, the bactericidal activity of EPO is enhanced, and after 24 h there are no viable cells. The similarity between the morphology of bacteria treated with EPO and the morphology of bacteria treated with EPO plus H2O2 suggests that the difference in bactericidal efficiency is quantitative rather than qualitative. Apart from the mechanisms of action of EPO, these findings suggest that in vivo, where hydrogen peroxide is degraded by a variety of mechanisms by both host cells and pathogens, EPO is expected to be more active than MPO; in fact, the latter enzyme does not show anti-M. tuberculosis activity in the absence of exogenously added H2O2.
We believe that it is it unlikely that toxic components other than EPO present in eosinophilic granules (22) can be responsible for the activity described above for the following reasons. First, our EPO preparation was highly pure, almost exhibiting the highest Rz reported to date, and produced a single band in SDS-PAGE gels. Second, our EPO preparation was devoid of RNase activity (data not shown), showing that two major toxic components of the eosinophilic granules, eosinophil cationic protein and eosinophil-derived neurotoxin, both members of the RNase superfamily (47), were either absent or present at negligible levels.
It is noteworthy that after short incubation times in the absence of H2O2, EPO killed no more than 80%of the M. tuberculosis cells. Increasing the incubation time did not significantly improve this activity. Therefore, the M. tuberculosis population might be heterogeneous in terms of susceptibility to EPO killing activity, and it is unclear why some bacteria may be resistant to EPO. One possibility is that the amount of enzyme which M. tuberculosis can bind to its surface varies among the cells in a bacterial population. In this case, bacteria heterogeneous for surface charge should be also heterogeneous for an EPO effect. In the presence of H2O2, all bacteria are killed. We speculate that during the first 2 h the population of bacteria killed corresponds to the population killed by EPO in the absence of H2O2. However, under these conditions, the activity is greater, and when the incubation time is increased, even those bacteria that resist the action of EPO alone succumb.
Some years ago, we demonstrated that human monocyte-derived macrophages can endocytose EPO at a higher efficiency than they can endocytose MPO. EPO-containing plasma membrane-borne vesicles may escape the fusion failure imposed by M. tuberculosis and reach the phagosome interior, as reported for other types of pinocytic vesicles (13, 42). Therefore, macrophages could be armed through extracellular EPO more efficiently than through MPO. EPO, either alone or in combination with H2O2 produced from the macrophage itself and discharged into the phagosome, can strongly improve the antimycobacterial activity of these phagocytes.
In conclusion, EPO offers various advantages over MPO for defining an antimycobacterial strategy. On a molar basis, EPO is as active against mycobacteria as MPO is, but EPO can work even in the absence of exogenously added H2O2. EPO is expected to reach the phagosome interior in the whole macrophage. This means that an EPO-containing macrophage may exhibit strong antimycobacterial activity. Experiments are under way to examine this hypothesis in in vivo models of tuberculosis in rabbits that are given aerosolized EPO and in cultures of either resident or activated human alveolar macrophages exposed to the purified enzyme.
Acknowledgments
This work was supported by Fondazione Carlo e Dirce Callerio ONLUS and by grants from Ministero dell'Università e della Ricerca Scientifica ex-60% and cofinanziamento ex-40% 1999.
We are grateful to John T. Belisle for the use of BSL-3 facilities (NIH, NIAID contract no. 1 AI-75320) at the Department of Microbiology, Immunology, and Pathology, Colorado State University, Fort Collins, Colo. We thank Tito Ubaldini and Claudio Gamboz for their collaboration in ultrastructural analysis and Maria Giovanna Perrotta for her technical assistance.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abu-Ghazaleh, R. I., S. L. Dunnette, D. A. Loegering, J. L. Checkel, H. Kita, L. L. Thomas, and G. J. Gleich. 1992. Eosinophil granule proteins in peripheral blood granulocytes. J. Leukoc. Biol. 52:611-618. [DOI] [PubMed] [Google Scholar]
- 2.Adreu, D., C. Carreno, C. Linde, H. G. Boman, and M. Andersson. 1999. Identification of an anti-mycobacterial domain in NK-lysin and granulysin. Biochem. J. 344:845-849. [PMC free article] [PubMed] [Google Scholar]
- 3.Appelberg, R. 1992. Interferon-gamma (IFN-γ) and macrophage inflammatory proteins (MIP-1 and MIP-2) are involved in the regulation of the T cell-dependent chronic peritoneal neutrophilia of mice infected with mycobacteria. Clin. Exp. Immunol. 89:269-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelberg, R. 1992. Macrophage inflammatory proteins MIP-1 and MIP-2 are involved in T cell mediated neutrophil recruitment. J. Leukoc. Biol. 52:303-306. [DOI] [PubMed] [Google Scholar]
- 5.Appelberg, R. 1992. Mycobacterial infection primes T cells and macrophages for enhanced recruitment of neutrophils. J. Leukoc. Biol. 51:472-477. [DOI] [PubMed] [Google Scholar]
- 6.Appelberg, R., and M. T. Silva. 1989. T cell dependent chronic neutrophilia during mycobacterial infections. Clin. Exp. Immunol. 78:478-483. [PMC free article] [PubMed] [Google Scholar]
- 7.Barker, R. L., R. H. Gundel, G. J. Gleich, J. L. Checkel, D. A. Loegering, L. R. Pease, and K. J. Hamann. 1991. Acidic polyamino acids inhibit human eosinophil granule major basic protein toxicity. J. Clin. Investig. 88:798-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borelli, V., E. Banfi, M. G. Perrotta, and G. Zabucchi. 1999. Myeloperoxidase exerts microbicidal activity against Mycobacterium tuberculosis. Infect. Immun. 67:4149-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, A. E., T. J. Holzer, and B. R. Andersen. 1987. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J. Infect. Dis. 156:985-991. [DOI] [PubMed] [Google Scholar]
- 10.Buys, J., R. Wever, and E. J. Ruitenberg. 1984. Myeloperoxidase is more efficient than eosinophil peroxidase in the in vitro killing of newborn larvae of Trichinella spiralis. Immunology 51:601-608. [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson, M. G., C. G. Peterson, and P. Venge. 1985. Human eosinophil peroxidase: purification and characterization. J. Immunol. 134:1875-1879. [PubMed] [Google Scholar]
- 12.Castro, A. G., N. Esaguy, P. M. Macedo, A. P. Aguas, and M. T. Silva. 1991. Live but not heat-killed mycobacteria cause rapid chemotaxis of large numbers of eosinophils in vivo and are ingested by the attracted granulocytes. Infect. Immun. 59:3009-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemens, D. L., and M. A. Horowitz. 1996. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administrated transferrin. J. Exp. Med. 184:1349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cramer, R., M. R. Soranzo, P. Dri, R. Menegazzi, A. Pitotti, G. Zabucchi, and P. Patriarca. 1984. A simple reliable assay for myeloperoxidase activity in mixed neutrophil-eosinophil cell suspensions: application to detection of myeloperoxidase deficiency. J. Immunol. Methods 70:119-125. [DOI] [PubMed] [Google Scholar]
- 15.Cramer, R., M. R. Soranzo, and P. Patriarca. 1981. Evidence that eosinophils catalyze the bromide-dependent decarboxylation of amino acids. Blood 58:1112-1118. [PubMed] [Google Scholar]
- 16.Dannenberg, A. M., and G. A. Rook. 1994. Pathogenesis of pulmonary tuberculosis: an interplay of tissue-damaging and macrophage-activating immune responses—dual mechanisms that control bacillary multiplication, p. 459-483. In B. R. Bloom (ed.),Tuberculosis: pathogenesis, protection and control. American Society for Microbiology, Washington, D.C.
- 17.Denis, M. 1991. Human neutrophils, activated with cytokines or not, do not kill virulent Mycobacterium tuberculosis. J. Infect. Dis. 163:919-925. [DOI] [PubMed] [Google Scholar]
- 18.Elsbach, P., J. Weiss, and O. Levy. 1999. Oxygen-independent antimicrobial systems of phagocytes, p. 801-817. In J. I. Gallin and R. Snyderman (ed.), Inflammation: basic principles and clinical correlates, 3rd ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 19.Ernst, W. A., S. Thoma-Uszynsky, R. Teitelbaum, C. Ko, D. A. Hanson, C. Clayberg, A. M. Krensky, M. Leippe, B. R. Bloom, T. Ganz, and R. L. Modlin. 2000. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J. Immunol. 165:7102-7108. [DOI] [PubMed] [Google Scholar]
- 20.Green, R. M., A. Seth, and N. D. Connell. 2000. A peptide permease mutant of Mycobacterium bovis BCG resistant to the toxic peptides glutathione and S-nitrosoglutathione. Infect. Immun. 68:429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hackam, D. J., O. D. Rotstein, W. Zhang, S. Gruenheid, P. Gros, and S. Grinstein. 1998. Host resistance to intracellular infection: mutation of natural resistance-associated macrophage protein 1 (Nramp1) impairs phagosomal acidification. J. Exp. Med. 188:351-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamann, K. J., R. L. Barker, R. M. Ten, and G. J. Gleich. 1991. The molecular biology of eosinophil granule proteins. Int. Arch. Allergy Appl. Immunol. 94:202-209. [DOI] [PubMed] [Google Scholar]
- 23.Hamann, K. J., G. J. Gleich, J. L. Checkel, D. A. Loegering, J. W. McCall, and R. L. Barker. 1990. In vitro killing of microfilariae of Brugia pahangi and Brugia malayi by eosinophil granule proteins. J. Immunol. 144:3166-3173. [PubMed] [Google Scholar]
- 24.Jong, E. C., W. R. Henderson, and S. J. Klebanoff. 1980. Bactericidal activity of eosinophil peroxidase. J. Immunol. 124:1378-1382. [PubMed] [Google Scholar]
- 25.Jong, E. C., and S. J. Klebanoff. 1980. Eosinophil-mediated mammalian tumor cell cytotoxicity: role of the peroxidase system. J. Immunol. 124:1949-1953. [PubMed] [Google Scholar]
- 26.Kaufmann, S. H. E. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11:129-163. [DOI] [PubMed] [Google Scholar]
- 27.Kirman, J., Z. Zakaria, K. McCoy, B. Delahunt, and G. Le Gros. 2000. Role of eosinophils in the pathogenesis of Mycobacterium bovis BCG infection in gamma interferon receptor-deficient mice. Infect. Immun. 68:2976-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klebanoff, S. J., J. M. Agosti, A. Jorg, and A. M. Waltersdorph. 1989. Comparative toxicity of the horse eosinophil peroxidase-H2O2-halide system and granule basic proteins. J. Immunol. 143:239-244. [PubMed] [Google Scholar]
- 29.Klebanoff, S. J., and C. C. Shepard. 1984. Toxic effect of the peroxidase-hydrogen peroxide-halide antimicrobial system on Mycobacterium leprae. Infect. Immun. 44:534-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lungarella, G., R. Menegazzi, C. Gardi, P. Spessotto, M. M. de Santi, P. Bertoncin, P. Patriarca, P. Calzoni, and G. Zabucchi. 1992. Identification of elastase in human eosinophils: immunolocalization, isolation, and partial characterization. Arch. Biochem. Biophys. 292:128-135. [DOI] [PubMed] [Google Scholar]
- 31.May, M. E., and P. J. Spagnuolo. 1987. Evidence for activation of the respiratory burst in the interaction of human neutrophils with Mycobacterium tuberculosis. Infect. Immun. 55:2304-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McWhinney, P. H., M. Yates, H. G. Prentice, M. Thrussell, S. H. Gillespie, and C. C. Kibbler. 1992. Infection caused by Mycobacterium chelonae: a diagnostic and therapeutic problem in the neutropenic patient. Clin. Infect. Dis. 14:1208-1212. [DOI] [PubMed] [Google Scholar]
- 33.Menegazzi, R., G. Zabucchi, A. Knowles, R. Cramer, and P. Patriarca. 1992. A new, one-step assay on whole cell suspensions for peroxidase secretion by human neutrophils and eosinophils. J. Leukoc. Biol. 52:619-624. [DOI] [PubMed] [Google Scholar]
- 34.Menegazzi, R., G. Zabucchi, and P. Patriarca. 1986. A simple procedure for the purification of eosinophil peroxidase from normal human blood. J. Immunol. Methods 91:283-288. [DOI] [PubMed] [Google Scholar]
- 35.Miyakawa, Y., P. Ratnakar, A. G. Rao, M. L. Costello, O. Mathieu-Costello, R. I. Lehrer, and A. Catanzaro. 1996. In vitro activity of the antimicrobial peptides human and rabbit defensins and porcine leukocyte protregrin against Mycobacterium tuberculosis. Infect. Immun. 64:926-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motojima, S., E. Frigas, D. A. Loegering, and G. J. Gleich. 1989. Toxicity of eosinophil cationic proteins for guinea pig tracheal epithelium in vitro. Am. Rev. Respir. Dis. 139:801-805. [DOI] [PubMed] [Google Scholar]
- 37.N′Diaye, E.-N., X. Darzacq, C. Astaire-Dequeker, M. Daffé, J. Calafat, and I. Maridonneau-Parini. 1998. Fusion of azurophil granules with phagosomes and activation of the tyrosine kinase Hck are specifically inhibited during phagocytosis of mycobacteria by human neutrophils. J. Immunol. 161:4983-4991. [PubMed] [Google Scholar]
- 38.Ogata, K., B. A. Linzer, R. I. Zuberi, T. Ganz, R. I. Lehrer, and A. Catanzaro. 1992. Activity of defensins from human neutrophilic granulocytes against Mycobacterium avium-Mycobacterium intracellulare. Infect. Immun. 60:4720-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedrosa, J., B. M. Saunders, R. Appelberg, I. M. Orme, M. T. Silva, and A. M. Cooper. 2000. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect. Immun. 68:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riedel, D. D., and S. H. E. Kaufmann. 1997. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect. Immun. 65:4620-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romeo, D., R. Cramer, T. Marzi, M. R. Soranzo, G. Zabucchi, and F. Rossi. 1973. Peroxidase activity of alveolar and peritoneal macrophages. J. Reticuloendothel. Soc. 13:399-409. [PubMed] [Google Scholar]
- 42.Russel, D. G., J. Dant, and S. Sturgill-Koszycki. 1996. Mycobacterium avium and Mycobacterium tuberculosis containing vacuoles are dynamic, fusion-competent vesicles that are accessible to glycosphingolipids from the host cell plasmalemma. J. Immunol. 156:4764-4773. [PubMed] [Google Scholar]
- 43.Sharma, S., I. Verma, and G. K. Khuller. 1999. Biochemical interaction of human neutrophil peptide-1 with Mycobacteirum tuberculosis H37Ra. Arch. Microbiol. 171:338-342. [DOI] [PubMed] [Google Scholar]
- 44.Sharma, S., I. Verma, and G. K. Khuller. 2000. Antibacterial activity of human neutrophil peptide-1 against Mycobacterium tuberculosis H37Rv: in vitro and ex vivo study. Eur. Respir. J. 16:112-117. [DOI] [PubMed] [Google Scholar]
- 45.Silva, M. T., M. N. T. Silva, and R. Appelberg. 1989. Neutrophil-macrophage cooperation in the host defense against mycobacterial infections. Microb. Pathog. 6:369-380. [DOI] [PubMed] [Google Scholar]
- 46.Slungaard, A., and J. R. Mahoney. 1991. Bromide-dependent toxicity of eosinophil peroxidase for endothelium and isolated working rat hearts: a model for eosinophilic endocarditis. J. Exp. Med. 173:117-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spry, C. J. F. 1988. Methods: eosinophil structure, constituents, and metabolism, p. 29-73. In Eosinophils: a comprehensive review, and guide to scientific and medical literature. Oxford University Press, Oxford, United Kingdom.
- 48.Stenger, S. D., A. Hanson, R. Teitlebaum, P. Dewan, K. R. Niazi, C. J. Froelich, T. Ganz, S. Thoma-Uszyinski, A. Melian, C. Bogdan, S. A. Porcelli, B. L. Bloom, A. M. Krensky, and R. L. Modlin. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282:121-125. [DOI] [PubMed] [Google Scholar]
- 49.Sturgill-Koszycki, S., P. H. Schlesinger, P. Chakraborty, P. L. Haddix, H. L. Collins, A. K. Fok, R. D. Allen, S. L. Gluck, J. Heuser, and D. G. Russell. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 11:678-681. [DOI] [PubMed] [Google Scholar]
- 50.Thomas, E. L., P. M. Bozeman, M. M. Jefferson, and C. C. King. 1995. Oxidation of bromide by the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase. Formation of bromamines. J. Biol. Chem. 270:2906-2913. [DOI] [PubMed] [Google Scholar]
- 51.Wever, R., H. Plat, and M. N. Hamers. 1981. Human eosinophil peroxidase: a novel isolation procedure, spectral properties and chlorinating activity. FEBS Lett. 123:327-331. [DOI] [PubMed] [Google Scholar]
- 52.Zabucchi, G., V. Borelli, M. G. Perrotta, M. R. Soranzo, M. Magnarin, R. Menegazzi, M. Romano, and F. Vita. 2001. Eosinophil peroxidase: structure, functions and perspectives. Recent Res. Dev. Allergy Clin. Immunol. 2:17-44. [Google Scholar]
- 53.Zabucchi, G., R. Menegazzi, R. Cramer, E. Nardon, and P. Patriarca. 1990. Mutual influence between eosinophil peroxidase (EPO) and neutrophils: neutrophils reversibly inhibit EPO enzymatic activity and EPO increases neutrophil adhesiveness. Immunology 69:580-587. [PMC free article] [PubMed] [Google Scholar]
- 54.Zabucchi, G., R. Menegazzi, M. R. Soranzo, and P. Patriarca. 1986. Uptake of human eosinophil peroxidase by human neutrophils. Am. J. Pathol. 124:510-518. [PMC free article] [PubMed] [Google Scholar]
- 55.Zabucchi, G., M. R. Soranzo, R. Menegazzi, P. Bertoncin, E. Nardon, and P. Patriarca. 1989. Uptake of human eosinophil peroxidase and myeloperoxidase by cells involved in the inflammatory process. J. Histochem. Cytochem. 37:499-508. [DOI] [PubMed] [Google Scholar]