Abstract
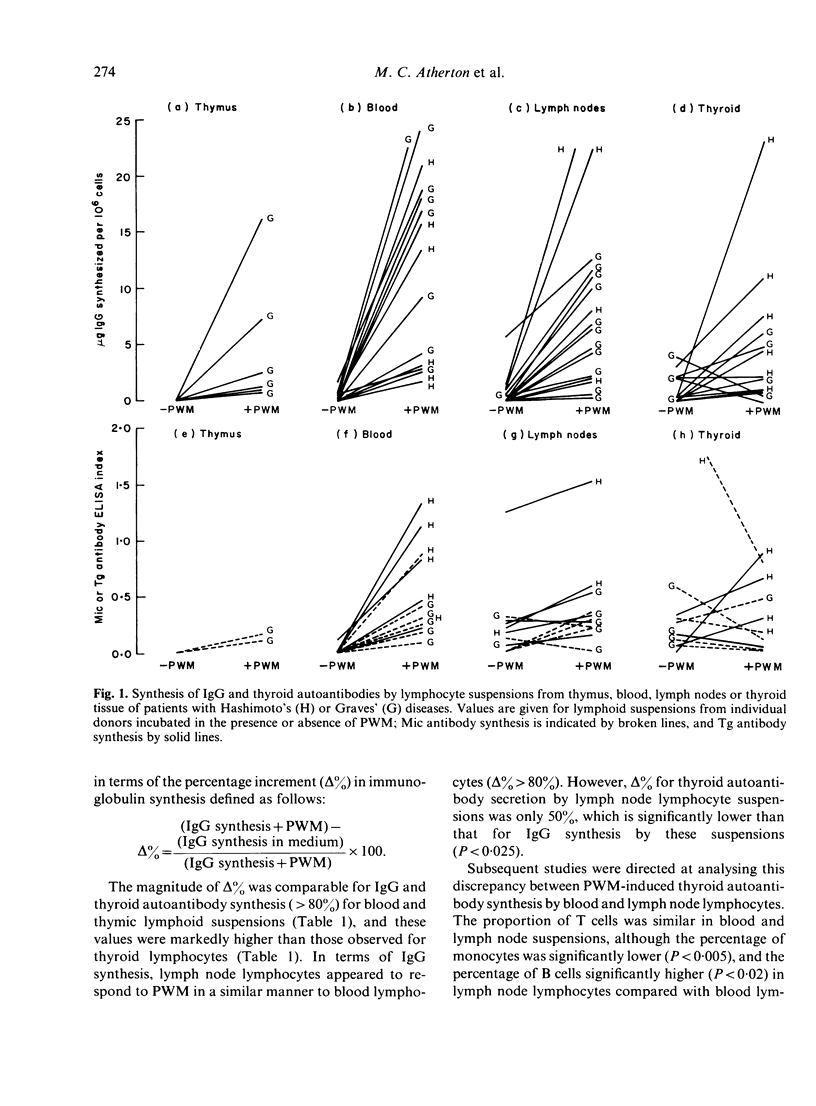
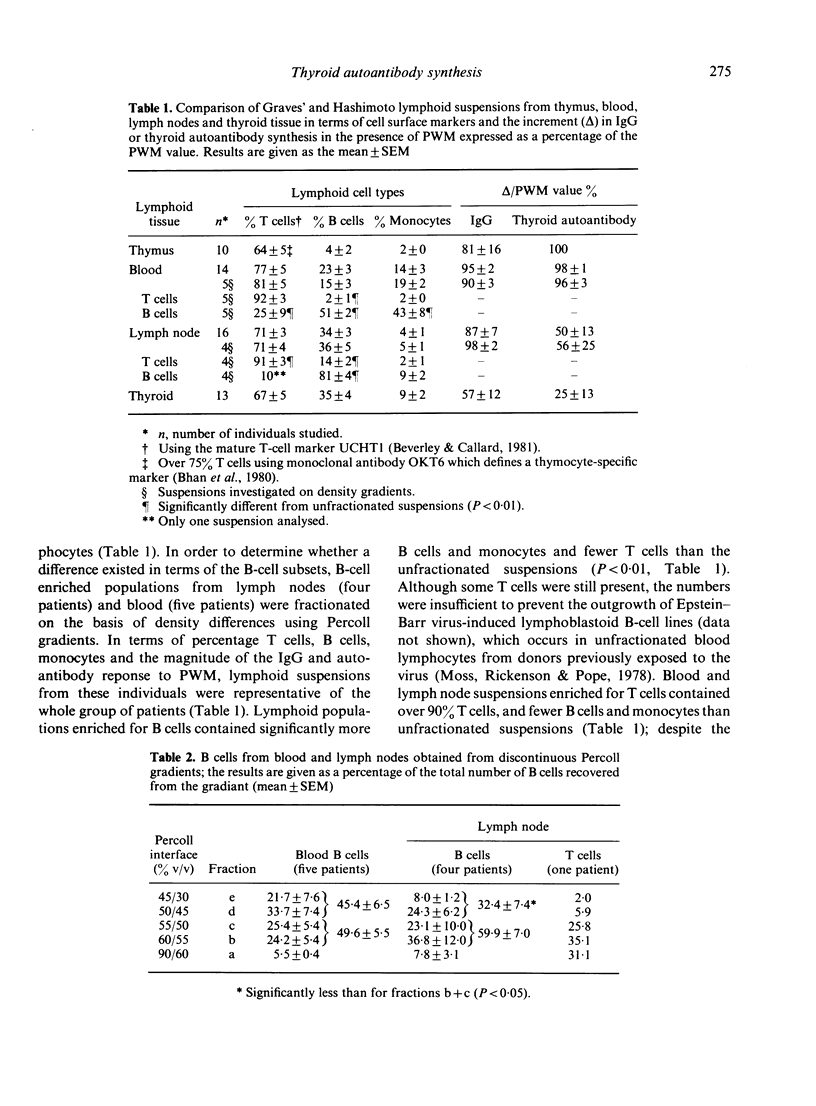
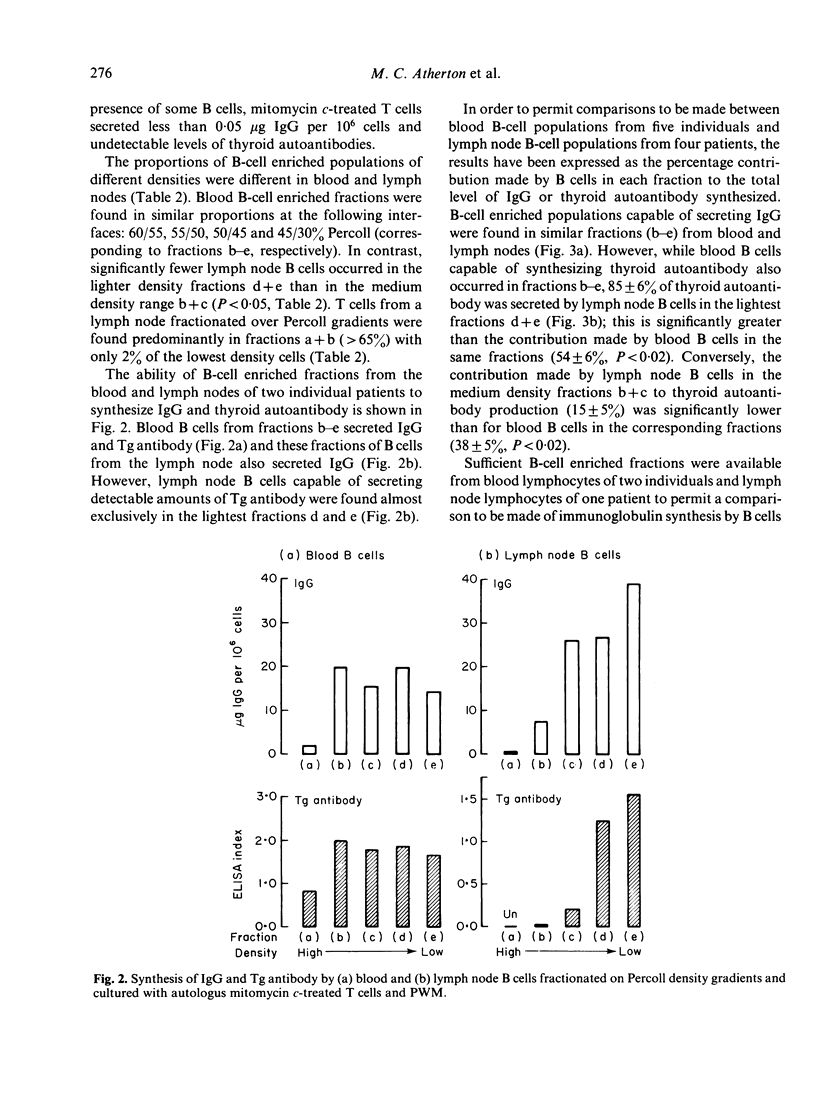
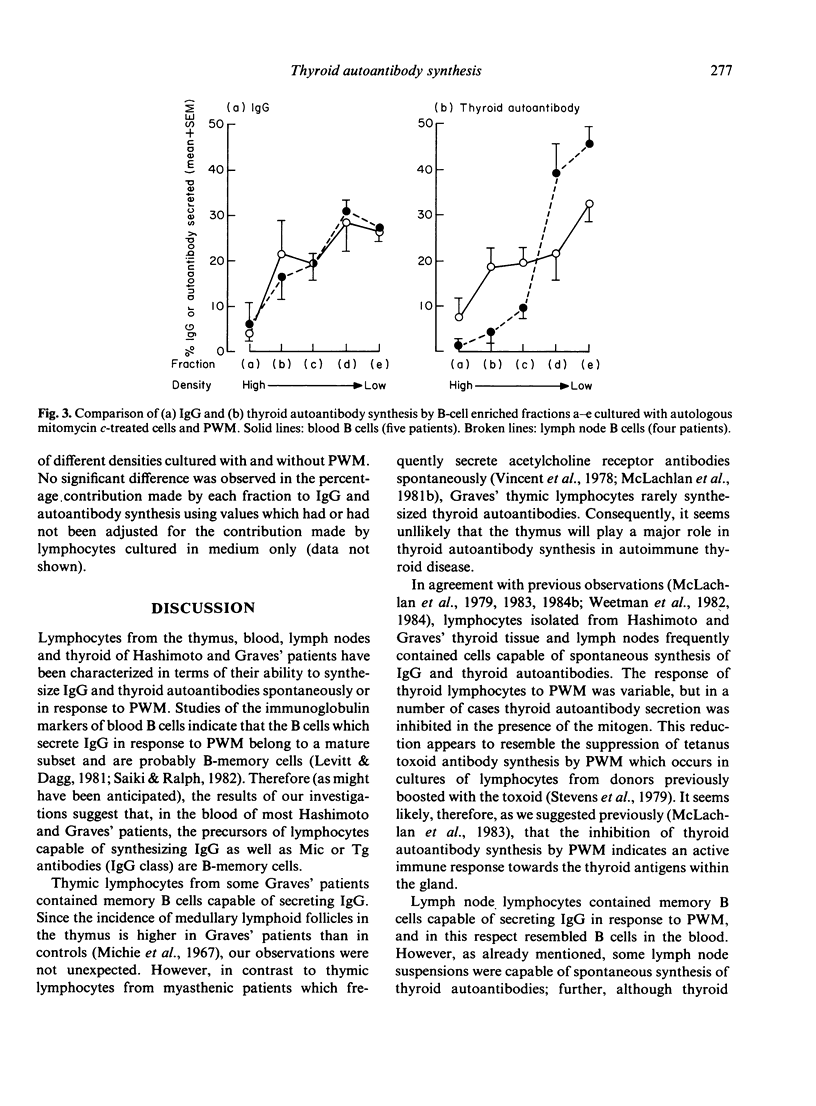
Lymphocytes from thymus, blood, lymph nodes and thyroid tissue of patients with autoimmune thyroid disease have been assessed for their ability to synthesize thyroid autoantibodies spontaneously or following stimulation by Pokeweed mitogen (PWM). Blood and thymic lymphocytes synthesized IgG and microsomal or thyroglobulin antibodies of IgG class in response to PWM (and were therefore probably B-memory cells), while thyroid lymphocytes frequently secreted autoantibodies spontaneously. Lymph node lymphocytes resembled blood lymphocytes in terms of increased production of IgG in response to PWM; however, spontaneous secretion of thyroid autoantibodies was observed in some lymph node suspensions, and the magnitude of the increment in thyroid autoantibodies synthesized in response to PWM was lower than that observed for blood lymphocytes. Fractionation of B-cell enriched populations on density gradients and subsequent incubation of the fractions with T cells and PWM demonstrated that, whereas blood B cells capable of synthesizing autoantibody were found in both medium and low density fractions, lymph node precursors of thyroid autoantibody-secreting cells were associated almost exclusively with the light fractions. The presence in lymph nodes of small numbers of low density B cells, compared with a much higher proportion of the heterogeneous population capable of secreting IgG, could account for the discrepancy between the IgG and autoantibody response to PWM. Further, it seems likely that the density difference in the autoantibody precursor population of lymph nodes and blood is related to the difference in the state of activation of B cells in these lymphoid organs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYSE E. A., OLD L. J., CHOUROULINKOV I. CYTOTOXIC TEST FOR DEMONSTRATION OF MOUSE ANTIBODY. Methods Med Res. 1964;10:39–47. [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Reinherz E. L., Poppema S., McCluskey R. T., Schlossman S. F. Location of T cell and major histocompatibility complex antigens in the human thymus. J Exp Med. 1980 Oct 1;152(4):771–782. doi: 10.1084/jem.152.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonica G. W., Kabelitz D., Sjöberg O., Aigrain Y., Wigzell H. Density distribution profiles of T cells: TM, TG and TA cells and response patterns in autologous versus allogeneic MLRs. Scand J Immunol. 1982 Sep;16(3):243–248. doi: 10.1111/j.1365-3083.1982.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Dagg M. K., Levitt D. Human B-lymphocyte subpopulations. I. Differentiation of density-separated B lymphocytes. Clin Immunol Immunopathol. 1981 Oct;21(1):39–49. doi: 10.1016/0090-1229(81)90193-8. [DOI] [PubMed] [Google Scholar]
- Levitt D., Dagg M. K. Human B-lymphocyte subpopulations. II. Plasma cell differentiation of isotype-specific B lymphocytes from peripheral blood. Clin Immunol Immunopathol. 1981 Oct;21(1):50–61. doi: 10.1016/0090-1229(81)90194-x. [DOI] [PubMed] [Google Scholar]
- McLachlan S. M., Bird A. G., Weetman A. P., Smith R., Hall R. Use of plaque assays to study thyroglobulin autoantibody synthesis by human peripheral blood lymphocytes. Scand J Immunol. 1981 Sep;14(3):233–242. doi: 10.1111/j.1365-3083.1981.tb00560.x. [DOI] [PubMed] [Google Scholar]
- McLachlan S. M., Clark S., Stimson W. H., Clark F., Smith B. R. Studies of thyroglobulin autoantibody synthesis using a micro-ELISA assay. Immunol Lett. 1982 Jan;4(1):27–33. doi: 10.1016/0165-2478(82)90073-6. [DOI] [PubMed] [Google Scholar]
- McLachlan S. M., McGregor A., Smith B. R., Hall R. Thyroid-autoantibody synthesis by Hashimoto thyroid lymphocytes. Lancet. 1979 Jan 20;1(8108):162–163. doi: 10.1016/s0140-6736(79)90559-2. [DOI] [PubMed] [Google Scholar]
- McLachlan S. M., Nicholson L. V., Venables G., Mastalgia F. L., Bates D., Smith B. R., Hall R. Acetylcholine receptor antibody synthesis in lymphocyte cultures. J Clin Lab Immunol. 1981 May;5(3):137–142. [PubMed] [Google Scholar]
- McLachlan S. M., Smith B. R., Hall R. Kinetics of immunoglobulin production by cultured human peripheral blood lymphocytes. J Immunol Methods. 1978;21(3-4):211–216. doi: 10.1016/0022-1759(78)90147-3. [DOI] [PubMed] [Google Scholar]
- Michie W., Beck J. S., Mahaffy R. G., Honein E. F., Fowler G. B. Quantitative radiological and histological studies of the thymus in thyroid disease. Lancet. 1967 Apr 1;1(7492):691–695. doi: 10.1016/s0140-6736(67)92177-0. [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Rickinson A. B., Pope J. H. Long-term T-cell-mediated immunity to Epstein-Barr virus in man. I. Complete regression of virus-induced transformation in cultures of seropositive donor leukocytes. Int J Cancer. 1978 Dec;22(6):662–668. doi: 10.1002/ijc.2910220604. [DOI] [PubMed] [Google Scholar]
- Nathanson S. D., Zamfirescu P. L., Drew S. I., Wilbur S. Two-step separation of human peripheral blood monocytes on discontinuous density gradients of colloidal silica-polyvinylpyrrolidinone. J Immunol Methods. 1977;18(3-4):225–234. doi: 10.1016/0022-1759(77)90176-4. [DOI] [PubMed] [Google Scholar]
- Saiki O., Ralph P. IgM- and IgD-bearing peripheral blood lymphocytes differentiate to IgM but not IgG or IgA immunoglobulin-secreting cells. Eur J Immunol. 1982 Jun;12(6):506–510. doi: 10.1002/eji.1830120611. [DOI] [PubMed] [Google Scholar]
- Schardt C. W., McLachlan S. M., Matheson J., Smith B. R. An enzyme-linked immunoassay for thyroid microsomal antibodies. J Immunol Methods. 1982 Dec 17;55(2):155–168. doi: 10.1016/0022-1759(82)90028-x. [DOI] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M. Enhancement by irradiated T cells of human plasma cell production: dissection of helper and suppressor functions in vitro. J Immunol. 1977 Feb;118(2):642–647. [PubMed] [Google Scholar]
- Vincent A., Scadding G. K., Thomas H. C., Newsom-Davis J. In-vitro synthesis of anti-acetylcholine-receptor antibody by thymic lymphocytes in myasthenia gravis. Lancet. 1978 Feb 11;1(8059):305–307. doi: 10.1016/s0140-6736(78)90073-9. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., McGregor A. M., Lazarus J. H., Hall R. Thyroid antibodies are produced by thyroid-derived lymphocytes. Clin Exp Immunol. 1982 Apr;48(1):196–200. [PMC free article] [PubMed] [Google Scholar]
- Weetman A. P., McGregor A. M., Wheeler M. H., Hall R. Extrathyroidal sites of autoantibody synthesis in Graves' disease. Clin Exp Immunol. 1984 May;56(2):330–336. [PMC free article] [PubMed] [Google Scholar]
- de Vere Tyndall A., Knight S. C., Edwards A. J., Clarke J. B. Veiled (dendritic) cells in synovial fluid. Lancet. 1983 Feb 26;1(8322):472–473. doi: 10.1016/s0140-6736(83)91468-x. [DOI] [PubMed] [Google Scholar]


