Abstract
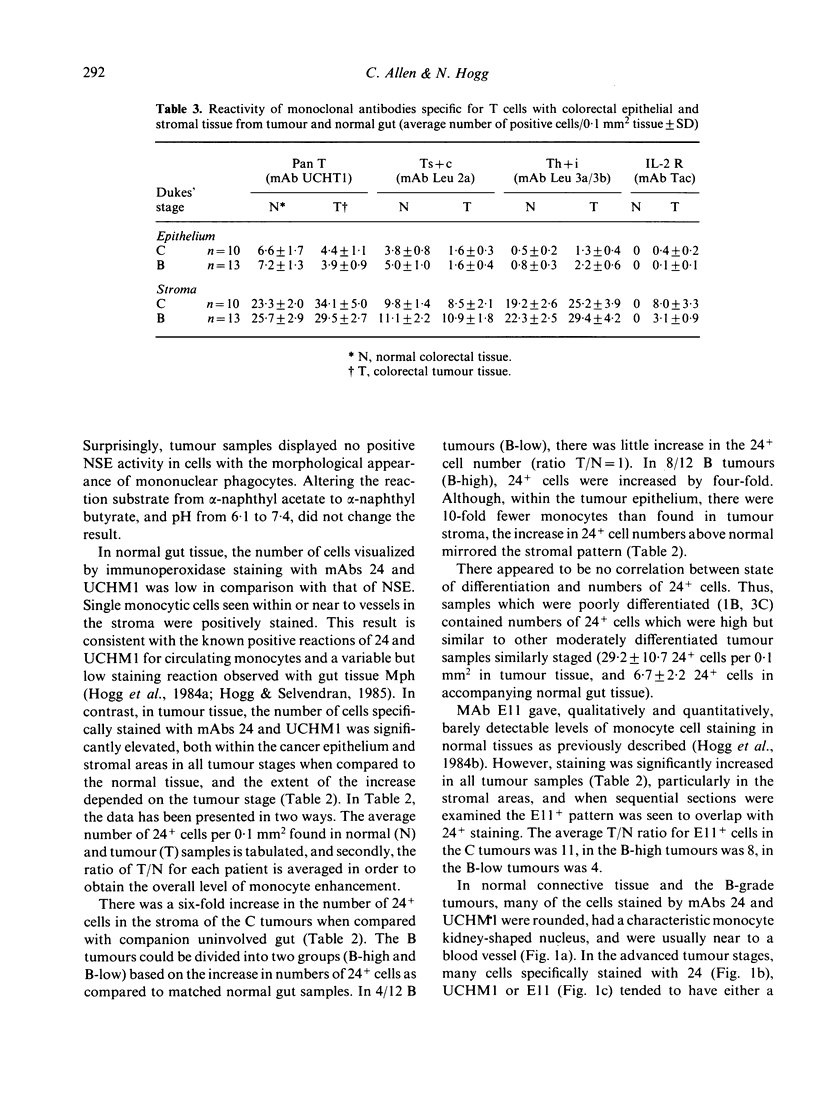
Monoclonal antibodies have been used to examine the patterns of infiltrating cells in colorectal tumours staged according to Dukes' classification. MAbs reacting with monocytes, but not tissue Mph, revealed a six-fold increase in monocytes in metastasizing C tumours compared to normal gut. The non-metastasizing B tumours could be divided into one group containing increased numbers of monocytes, and a second group comparable to control gut. T-cell numbers were increased in all tumour stages by an average 1.4-fold, which disguised the lack of consistent pattern in T-cell subset ratios in the tumour stromal tissue. However, in the tumour epithelium, there was a constant decrease in the Ts + c cell subset and a subsequent alteration in T-cell subset ratio in favour of Th + i cells. With the progression from Dukes' Stage B to C, there was an increase in the proportion of monocytes and T cells which were activated as detected by mAbs to the C3b receptor and IL-2 receptor, respectively. These observations suggest that an immune response is in progress in these colorectal tumours and that it is most active in the metastasizing Dukes' C tumours. Whether this response is elicited by the tumour or other elements, whether it is detrimental to tumour growth, or whether it is actively assisting tumour growth and possibly dissemination, are matters of conjecture.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., DesMarais C. L. Immunohistologic characterization of major histocompatibility antigens and inflammatory cellular infiltrate in human breast cancer. J Natl Cancer Inst. 1983 Sep;71(3):507–516. [PubMed] [Google Scholar]
- Cerf-Bensussan N., Schneeberger E. E., Bhan A. K. Immunohistologic and immunoelectron microscopic characterization of the mucosal lymphocytes of human small intestine by the use of monoclonal antibodies. J Immunol. 1983 Jun;130(6):2615–2622. [PubMed] [Google Scholar]
- DUKES C. E., BUSSEY H. J. The spread of rectal cancer and its effect on prognosis. Br J Cancer. 1958 Sep;12(3):309–320. doi: 10.1038/bjc.1958.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Glickman E., Evans R. L. Antibodies to membrane structures that distinguish suppressor/cytotoxic and helper T lymphocyte subpopulations block the mixed leukocyte reaction in man. J Exp Med. 1981 Jul 1;154(1):193–198. doi: 10.1084/jem.154.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. Host cells in transplanted murine tumors and their possible relevance to tumor growth. J Reticuloendothel Soc. 1979 Oct;26(4):427–437. [PubMed] [Google Scholar]
- Fearon D. T., Collins L. A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983 Jan;130(1):370–375. [PubMed] [Google Scholar]
- Hogg N., MacDonald S., Slusarenko M., Beverley P. C. Monoclonal antibodies specific for human monocytes, granulocytes and endothelium. Immunology. 1984 Dec;53(4):753–767. [PMC free article] [PubMed] [Google Scholar]
- Hogg N., Ross G. D., Jones D. B., Slusarenko M., Walport M. J., Lachmann P. J. Identification of an anti-monocyte monoclonal antibody that is specific for membrane complement receptor type one (CR1). Eur J Immunol. 1984 Mar;14(3):236–243. doi: 10.1002/eji.1830140307. [DOI] [PubMed] [Google Scholar]
- Kadhim S. A., Rees R. C. Enhancement of tumor growth in mice: evidence for the involvement of host macrophages. Cell Immunol. 1984 Aug;87(1):259–269. doi: 10.1016/0008-8749(84)90150-3. [DOI] [PubMed] [Google Scholar]
- Kornstein M. J., Brooks J. S., Elder D. E. Immunoperoxidase localization of lymphocyte subsets in the host response to melanoma and nevi. Cancer Res. 1983 Jun;43(6):2749–2753. [PubMed] [Google Scholar]
- Lauder I., Aherne W., Stewart J., Sainsbury R. Macrophage infiltration of breast tumours: a prospective study. J Clin Pathol. 1977 Jun;30(6):563–568. doi: 10.1136/jcp.30.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A. Effects on in vitro tumor growth of murine macrophages isolated from sarcoma lines differing in immunogenicity and metastasizing capacity. Int J Cancer. 1978 Dec;22(6):741–746. doi: 10.1002/ijc.2910220617. [DOI] [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Sekaly P. R., Moretta A., Chapuis B., Cerottini J. C. Surface markers of cloned human T cells with various cytolytic activities. J Exp Med. 1981 Aug 1;154(2):569–574. doi: 10.1084/jem.154.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn R. T. Immunostimulation of the lymphodependent phase of neoplastic growth. J Natl Cancer Inst. 1977 Oct;59(4):1043–1049. doi: 10.1093/jnci/59.4.1043. [DOI] [PubMed] [Google Scholar]
- Pretlow T. P., Keith E. F., Cryar A. K., Bartolucci A. A., Pitts A. M., Pretlow T. G., 2nd, Kimball P. M., Boohaker E. A. Eosinophil infiltration of human colonic carcinomas as a prognostic indicator. Cancer Res. 1983 Jun;43(6):2997–3000. [PubMed] [Google Scholar]
- Rognum T. O., Brandtzaeg P., Baklien K., Hognestad J. Immunoglobulin-producing cells in the "transitional" mucosa adjacent to adenocarcinomas of the human large bowel. Int J Cancer. 1979 Feb;23(2):165–173. doi: 10.1002/ijc.2910230205. [DOI] [PubMed] [Google Scholar]
- Ruiter D. J., Bhan A. K., Harrist T. J., Sober A. J., Mihm M. C., Jr Major histocompatibility antigens and mononuclear inflammatory infiltrate in benign nevomelanocytic proliferations and malignant melanoma. J Immunol. 1982 Dec;129(6):2808–2815. [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Lymphocyte subpopulations in the human small intestine. The findings in normal mucosa and in the mucosa of patients with adult coeliac disease. Clin Exp Immunol. 1983 Apr;52(1):219–228. [PMC free article] [PubMed] [Google Scholar]
- Svennevig J. L., Lunde O. C., Holter J. In situ analysis of the inflammatory cell infiltrates in colon carcinomas and in the normal colon wall. Acta Pathol Microbiol Immunol Scand A. 1982 Mar;90(2):131–137. doi: 10.1111/j.1699-0463.1982.tb00073_90a.x. [DOI] [PubMed] [Google Scholar]
- Svennevig J. L., Svaar H. Content and distribution of macrophages and lymphocytes in solid malignant human tumours. Int J Cancer. 1979 Dec 15;24(6):754–758. doi: 10.1002/ijc.2910240609. [DOI] [PubMed] [Google Scholar]
- Thomas Y., Rogozinski L., Irigoyen O. H., Friedman S. M., Kung P. C., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. IV. Induction of suppressor cells within the OKT4+ population. J Exp Med. 1981 Aug 1;154(2):459–467. doi: 10.1084/jem.154.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Underwood J. C. Lymphoreticular infiltration in human tumours: prognostic and biological implications: a review. Br J Cancer. 1974 Dec;30(6):538–548. doi: 10.1038/bjc.1974.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G. W., Gollahon K. A. Detection and quantitation of macrophage infiltration into primary human tumors with the use of cell-surface markers. J Natl Cancer Inst. 1977 Oct;59(4):1081–1087. doi: 10.1093/jnci/59.4.1081. [DOI] [PubMed] [Google Scholar]
- Wood G. W., Gollahon K. A. T-lymphocytes and macrophages in primary murine fibrosarcomas at different stages in their progression. Cancer Res. 1978 Jul;38(7):1857–1865. [PubMed] [Google Scholar]
- Wright S. D., Craigmyle L. S., Silverstein S. C. Fibronectin and serum amyloid P component stimulate C3b- and C3bi-mediated phagocytosis in cultured human monocytes. J Exp Med. 1983 Oct 1;158(4):1338–1343. doi: 10.1084/jem.158.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]




