Abstract
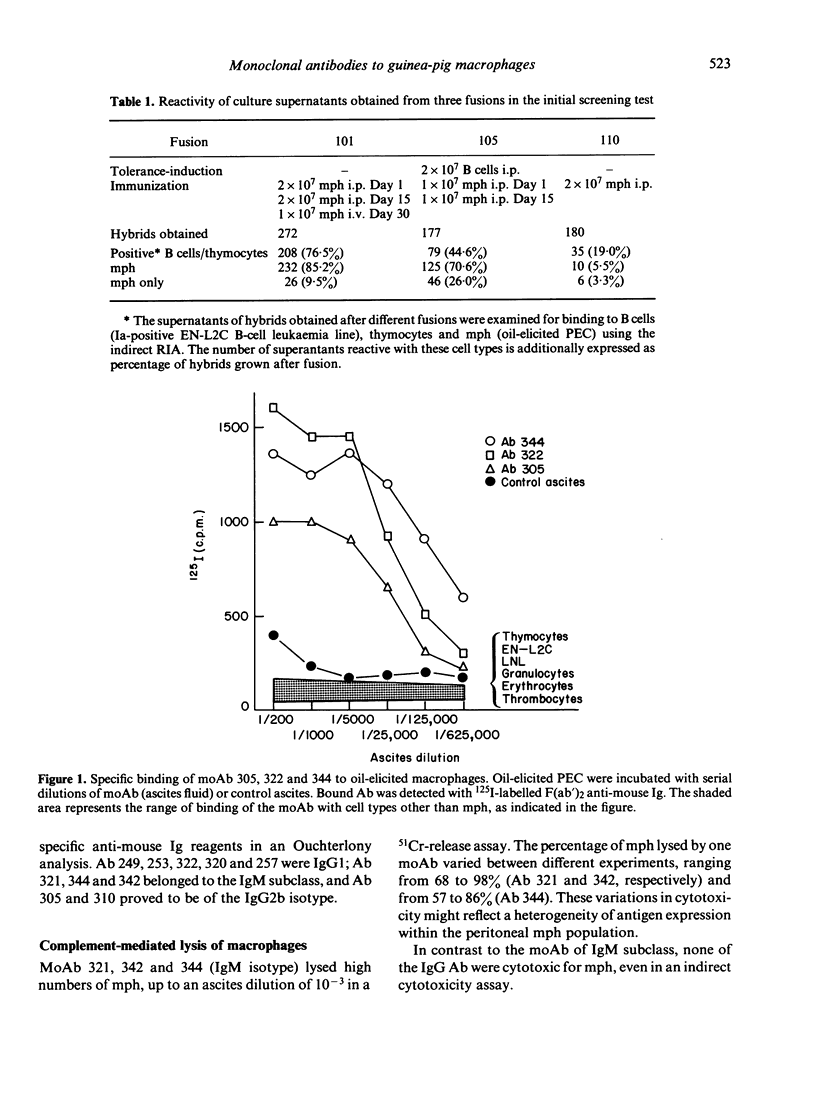
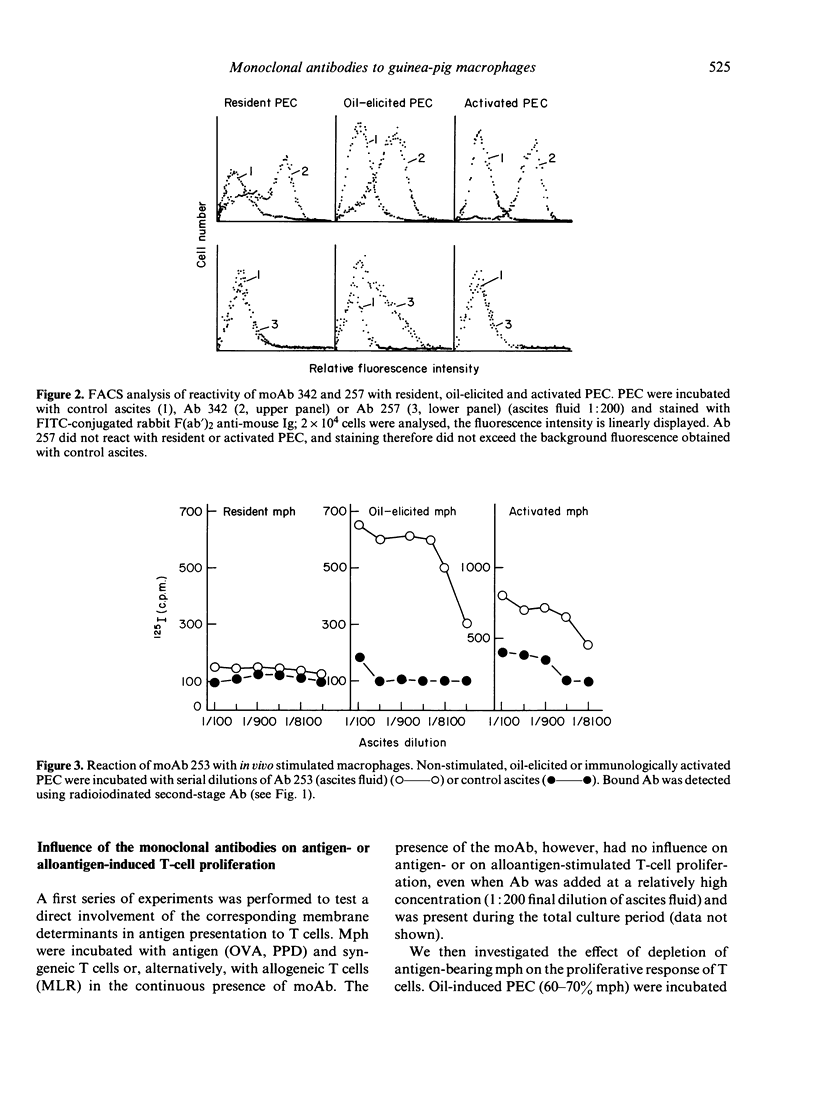
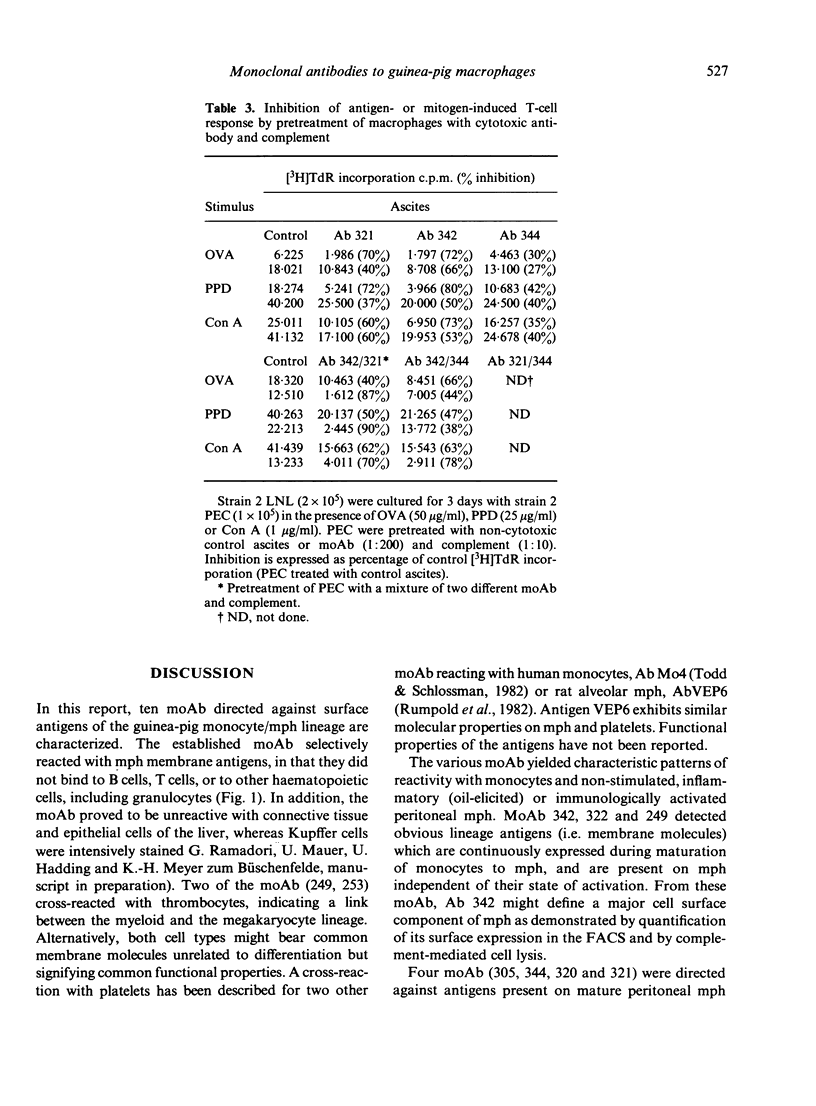
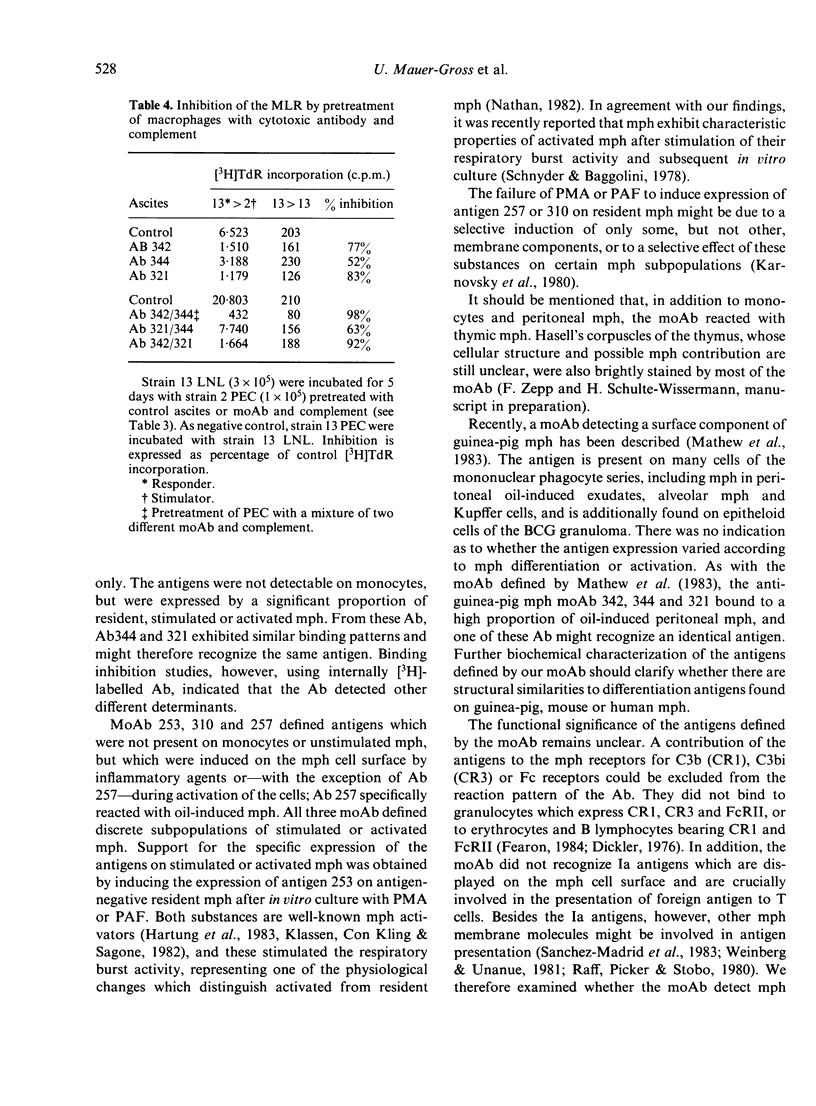
Ten monoclonal antibodies (moAb) directed against cell surface antigens of guinea-pig monocytes and macrophages (mph) were produced and characterized. The corresponding antigens are not present on granulocytes, T lymphocytes, an Ia-positive B-cell line or other haematopoietic cells. In binding or cytotoxicity assays, the moAb demonstrated characteristics patterns of reactivity, with mph being in different stages of differentiation or activation. Three moAb (342, 322, 249) recognized 'lineage antigens' (i.e. antigens continuously expressed during maturation of monocytes to mph and after stimulation or activation of the cells). MoAb 342 possibly defines a major cell surface determinant, being present on 90% of mph. The antigens detected by moAb 305, 320, 321 and 344 characterize mature mph. They were not expressed on monocytes, but were expressed on the majority of resident, elicited or activated peritoneal mph. MoAb 253, 310 or 257 defined discrete subpopulations of elicited and--with the exception of moAb 257--activated mph. The corresponding antigens were not present on monocytes or resident mph, but appeared on the cell surface during in vivo or in vitro stimulation of the cells. There was no indication of a contribution of the moAb-defined antigens to the presentation of antigen, mitogen or alloantigen by the mph to T cells. The functional significance of the antigens thus remains to be elucidated. Our studies indicate that cells committed to the monocyte/mph lineage share a family of differentiation antigens, distinguishing them from other cell lines. The moAb provide useful tools for further investigation of the activation of mph and allow the rapid detection of mph in different tissues.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger R., Clement L., Schroer J., Chiba J., Shevach E. M. Monoclonal antibodies to guinea pig Ia antigens. I. Production, serologic, and immunochemical characterization. J Immunol. 1981 Jan;126(1):32–37. [PubMed] [Google Scholar]
- Burger R., Shevach E. M. Monoclonal antibodies to guinea pig Ia antigens. II. Effect on alloantigen-, antigen-, and mitogen-induced T lymphocyte proliferation in vitro. J Exp Med. 1980 Oct 1;152(4):1011–1023. doi: 10.1084/jem.152.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement L. T., Hu C., Shevach E. M., Green I. Immunologic characterization of a granulocytic leukemia of inbred strain 13 guinea pigs: presence of Ia-positive myeloblasts. Blood. 1980 Feb;55(2):304–310. [PubMed] [Google Scholar]
- Dickler H. B. Lymphocyte receptors for immunoglobulin. Adv Immunol. 1976;24:167–214. doi: 10.1016/s0065-2776(08)60330-2. [DOI] [PubMed] [Google Scholar]
- Forni G., Shevach E. M., Green I. Mutant lines of guinea pig L2C leukemia. I. Deletion of Ia alloantigens is associated with a loss in immunogenicity of tumor-associated transplantation antigens. J Exp Med. 1976 May 1;143(5):1067–1081. doi: 10.1084/jem.143.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung H. P., Parnham M. J., Winkelmann J., Englberger W., Hadding U. Platelet activating factor (PAF) induces the oxidative burst in macrophages. Int J Immunopharmacol. 1983;5(2):115–121. doi: 10.1016/0192-0561(83)90002-4. [DOI] [PubMed] [Google Scholar]
- Hopper K. E., Geczy C. L. Characterization of guinea pig macrophages. I. Mobility and maturation of peritoneal cells following inflammatory stimuli. Cell Immunol. 1980 Dec;56(2):400–414. doi: 10.1016/0008-8749(80)90116-1. [DOI] [PubMed] [Google Scholar]
- Klassen D. K., Conkling P. R., Sagone A. L., Jr Activation of monocyte and granulocyte antibody-dependent cytotoxicity by phorbol myristate acetate. Infect Immun. 1982 Mar;35(3):818–825. doi: 10.1128/iai.35.3.818-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R. C., Katayama I., Gupta S. K., Curtis J., Turk J. L. Analysis of cells of the mononuclear phagocyte series in experimental mycobacterial granulomas by monoclonal antibodies. Infect Immun. 1983 Jan;39(1):344–352. doi: 10.1128/iai.39.1.344-352.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer U., Burger R., von Steldern D., Bitter-Suermann D., Hadding U. Expression of Ia antigens on macrophages is reduced after stimulation with homologous C3b. J Immunol. 1984 Jun;132(6):2802–2806. [PubMed] [Google Scholar]
- Mizel S. B., Ben-Zvi A. Studies on the role of lymphocyte-activating factor (Interleukin 1) in antigen-induced lymph node lymphocyte proliferation. Cell Immunol. 1980 Sep 1;54(2):382–389. doi: 10.1016/0008-8749(80)90218-x. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretion of oxygen intermediates: role in effector functions of activated macrophages. Fed Proc. 1982 Apr;41(6):2206–2211. [PubMed] [Google Scholar]
- Pollack V. A., Brandhorst J. S., Hanna M. G., Jr Separation of guinea pig peripheral blood lymphocytes by discontinuous density gradient centrifugation using Ficoll-metrizoate. J Immunol Methods. 1981;41(1):29–41. doi: 10.1016/0022-1759(81)90271-4. [DOI] [PubMed] [Google Scholar]
- Raff H. V., Picker L. J., Stobo J. D. Macrophage heterogeneity in man. A subpopulation of HLA-DR-bearing macrophages required for antigen-induced T cell activation also contains stimulators for autologous-reactive T cells. J Exp Med. 1980 Sep 1;152(3):581–593. doi: 10.1084/jem.152.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Rumpold H., Förster O., Böck G., Swetly P., Riedl M. Antigenic heterogeneity of rat macrophages. A monoclonal antibody reacting only with alveolar but not with other types of macrophages. Immunology. 1982 Apr;45(4):637–643. [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder J., Baggiolini M. Role of phagocytosis in the activation of macrophages. J Exp Med. 1978 Dec 1;148(6):1449–1457. doi: 10.1084/jem.148.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M., Rosenstreich D. L., Green I. The distribution of histocompatibility antigens on T and B cells in the guinea pig. Transplantation. 1973 Aug;16(2):126–133. doi: 10.1097/00007890-197308000-00008. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Gutchinov B., Witmer M. D., Nussenzweig M. C. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983 Feb 1;157(2):613–627. doi: 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Schlossman S. F. Analysis of antigenic determinants on human monocytes and macrophages. Blood. 1982 Apr;59(4):775–786. [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Weinberg D. S., Unanue E. R. Antigen-presenting function of alveolar macrophages: uptake and presentation of Listeria monocytogenes. J Immunol. 1981 Feb;126(2):794–799. [PubMed] [Google Scholar]


