Abstract
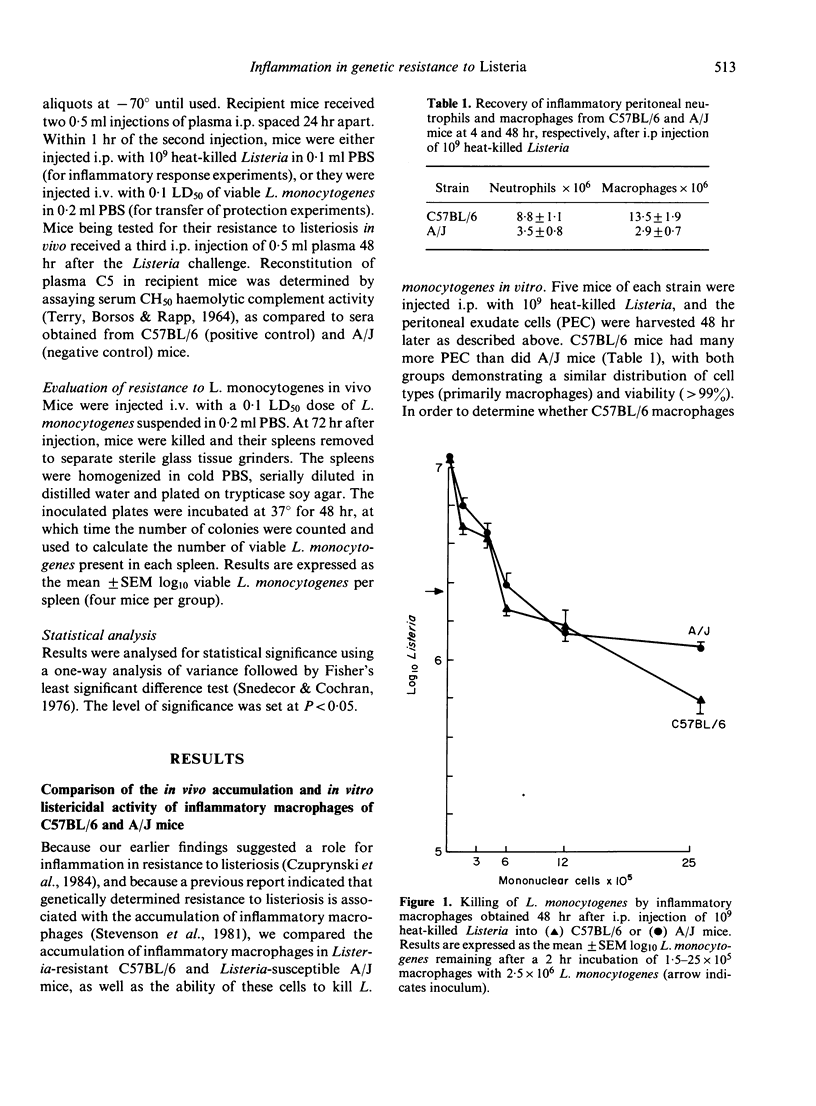
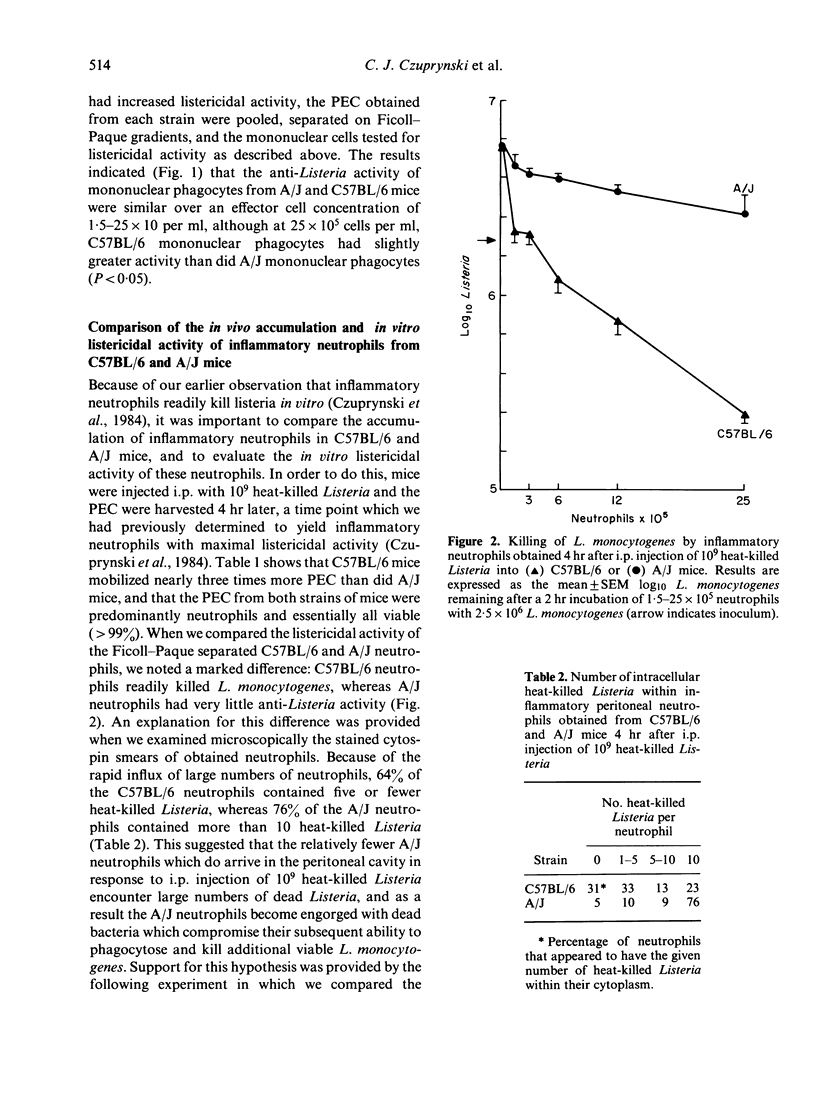
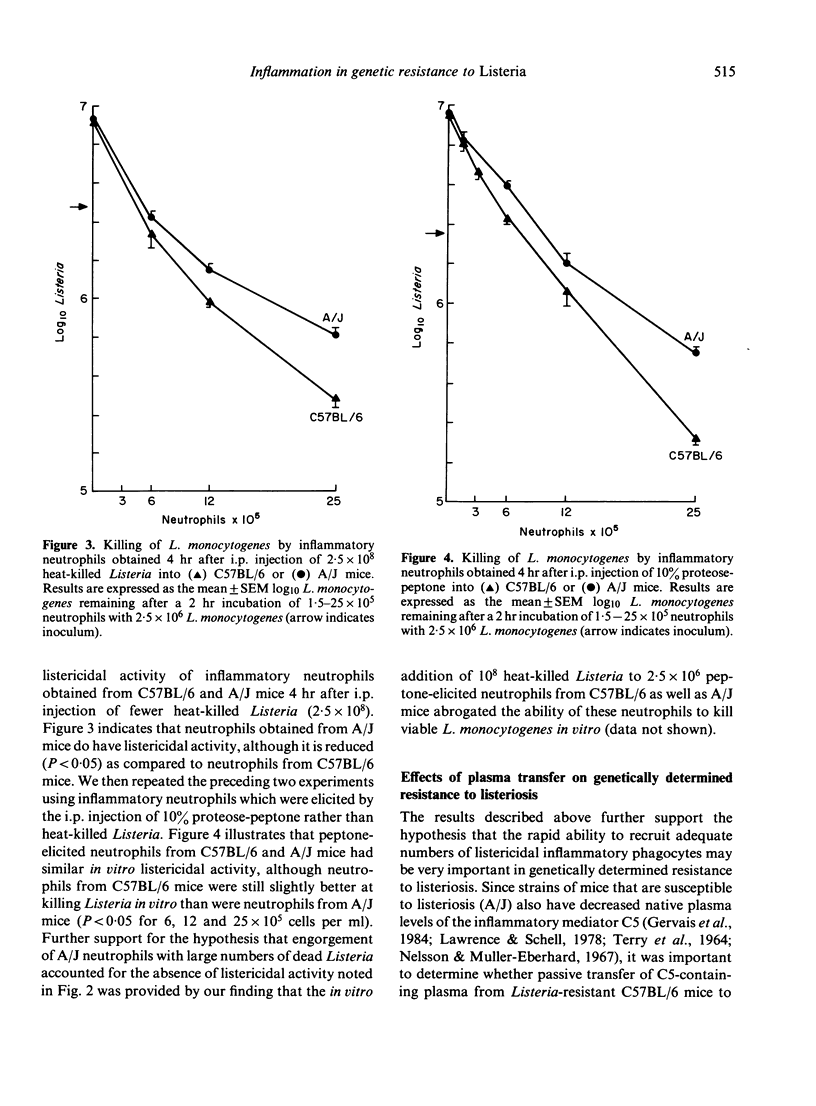
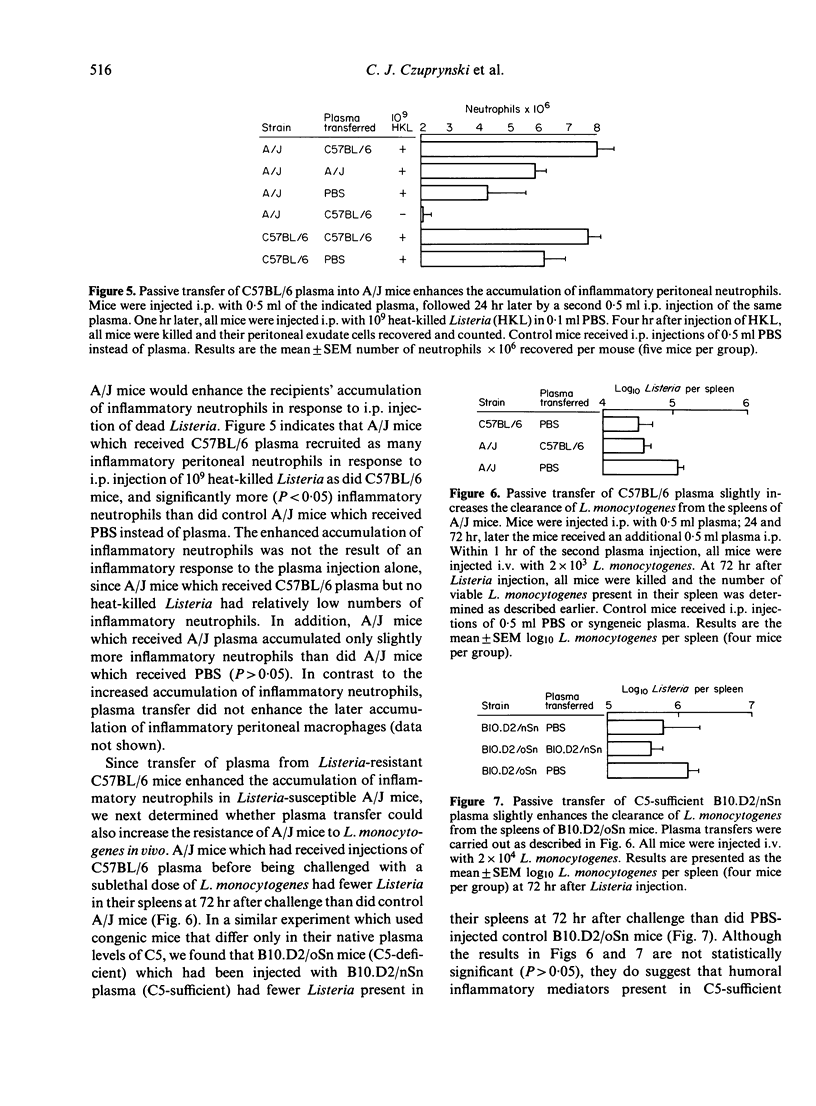
The C57BL/6 and A/J inbred strains of mice differ markedly in their resistance to the facultative intracellular bacterium Listeria monocytogenes. One possible explanation for this genetically determined resistance is that phagocytes from Listeria-resistant strains of mice can kill L. monocytogenes more effectively than phagocytes from Listeria-susceptible strains of mice. We report here that inflammatory neutrophils and macrophages from Listeria-resistant mice (C57BL/6) exhibit a slight but significantly enhanced ability to kill L. monocytogenes in vitro as compared to inflammatory phagocytes from Listeria-susceptible mice (A/J). More importantly, however, Listeria-resistant mice recruited more inflammatory neutrophils and macrophages to the peritoneal cavity in response to i.p. injection of heat-killed Listeria than did Listeria-susceptible mice. These data suggest that genetically determined resistance to listeriosis is dependent on the enhanced inflammatory responsiveness of Listeria-resistant mice. Further support for this hypothesis was provided by experiments in which the passive transfer to A/J mice (C5-deficient) of plasma from C57BL/6 mice (C5-sufficient) enhanced the ability of the recipient A/J mice both to recruit inflammatory neutrophils to the peritoneal cavity in response to i.p. injection of heat-killed Listeria, and to clear L. monocytogenes from the spleen after a sublethal challenge of viable Listeria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker L. A., Campbell P. A., Hollister J. R. Chemotaxigenesis and complement fixation by Listeria monocytogenes cell wall fractions. J Immunol. 1977 Nov;119(5):1723–1726. [PubMed] [Google Scholar]
- Campbell P. A., Czuprynski C. J., Cook J. L. Differential expression of macrophage effector functions: bactericidal versus tumoricidal activities. J Leukoc Biol. 1984 Sep;36(3):293–306. doi: 10.1002/jlb.36.3.293. [DOI] [PubMed] [Google Scholar]
- Caren L. D., Rosenberg L. T. The role of complement in resistance to endogenous and exogenous infection with a common mouse pathogen, Corynebacterium kutscheri. J Exp Med. 1966 Oct 1;124(4):689–699. doi: 10.1084/jem.124.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. doi: 10.1128/iai.19.3.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Campbell P. A., Henson P. M. Killing of Listeria monocytogenes by human neutrophils and monocytes, but not by monocyte-derived macrophages. J Reticuloendothel Soc. 1983 Jul;34(1):29–44. [PubMed] [Google Scholar]
- Czuprynski C. J., Henson P. M., Campbell P. A. Enhanced accumulation of inflammatory neutrophils and macrophages mediated by transfer of T cells from mice immunized with Listeria monocytogenes. J Immunol. 1985 May;134(5):3449–3454. [PubMed] [Google Scholar]
- Czuprynski C. J., Henson P. M., Campbell P. A. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J Leukoc Biol. 1984 Feb;35(2):193–208. doi: 10.1002/jlb.35.2.193. [DOI] [PubMed] [Google Scholar]
- Gervais F., Stevenson M., Skamene E. Genetic control of resistance to Listeria monocytogenes: regulation of leukocyte inflammatory responses by the Hc locus. J Immunol. 1984 Apr;132(4):2078–2083. [PubMed] [Google Scholar]
- Goodman M. G., Chenoweth D. E., Weigle W. O. Potentiation of the primary humoral immune response in vitro by C5a anaphylatoxin. J Immunol. 1982 Jul;129(1):70–75. [PubMed] [Google Scholar]
- Hugli T. E., Müller-Eberhard H. J. Anaphylatoxins: C3a and C5a. Adv Immunol. 1978;26:1–53. doi: 10.1016/s0065-2776(08)60228-x. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., Pepys M. B. Delayed hypersensitivity reactions to Listeria monocytogenes in rats decomplemented with cobra factor and in C5-deficient mice. Immunology. 1981 Jun;43(2):271–279. [PMC free article] [PubMed] [Google Scholar]
- Larsen G. L., McCarthy K., Webster R. O., Henson J., Henson P. M. A differential effect of C5a and C5a des Arg in the induction of pulmonary inflammation. Am J Pathol. 1980 Jul;100(1):179–192. [PMC free article] [PubMed] [Google Scholar]
- Lawrence D. A., Schell R. F. Susceptibility of C5-deficient mice to listeriosis: modulation by Concanavalin A. Cell Immunol. 1978 Sep;39(2):336–344. doi: 10.1016/0008-8749(78)90109-0. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel T. E., Cheers C. Resistance and susceptibility of mice to bacterial infection: histopathology of listeriosis in resistant and susceptible strains. Infect Immun. 1980 Dec;30(3):851–861. doi: 10.1128/iai.30.3.851-861.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen R. F., Beisel K., Zeleznik N. J., Le P. T. Acute-phase reactants of mice. II. Strain dependence of serum amyloid P-component (SAP) levels and response to inflammation. J Immunol. 1983 Feb;130(2):885–889. [PubMed] [Google Scholar]
- Nesbitt M. N., Skamene E. Recombinant inbred mouse strains derived from A/J and C57BL/6J: a tool for the study of genetic mechanisms in host resistance to infection and malignancy. J Leukoc Biol. 1984 Sep;36(3):357–364. doi: 10.1002/jlb.36.3.357. [DOI] [PubMed] [Google Scholar]
- Nilsson U. R., Müller-Eberhard H. J. Deficiency of the fifth component of complement in mice with an inherited complement defect. J Exp Med. 1967 Jan 1;125(1):1–16. doi: 10.1084/jem.125.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIERCE-CHASE C. H., FAUVE R. M., DUBOS R. CORYNEBACTERIAL PSEUDOTUBERCULOSIS IN MICE. I. COMPARATIVE SUSCEPTIBILITY OF MOUSE STRAINS TO EXPERIMENTAL INFECTION WITH CORYNEBACTERIUM KUTSCHERI. J Exp Med. 1964 Aug 1;120:267–281. doi: 10.1084/jem.120.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. C. Resistance to listeriosis in mice that are deficient in the fifth component of complement. Infect Immun. 1980 Jan;27(1):61–67. doi: 10.1128/iai.27.1.61-67.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamene E. Genetic regulation of host resistance to bacterial infection. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S823–S832. doi: 10.1093/clinids/5.supplement_4.s823. [DOI] [PubMed] [Google Scholar]
- Sluiter W., Elzenga-Claasen I., van der Voort van der Kley-van Andel A., van Furth R. Differences in the response of inbred mouse strains to the factor increasing monocytopoiesis. J Exp Med. 1984 Feb 1;159(2):524–536. doi: 10.1084/jem.159.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Shin H. S., Hausman M. H. A chemotactic factor for mononuclear leukocytes. Proc Soc Exp Biol Med. 1971 Nov;138(2):387–390. doi: 10.3181/00379727-138-35903. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Kongshavn P. A., Skamene E. Genetic linkage of resistance to Listeria monocytogenes with macrophage inflammatory responses. J Immunol. 1981 Aug;127(2):402–407. [PubMed] [Google Scholar]
- TERRY W. D., BORSOS T., RAPP H. J. DIFFERENCES IN SERUM COMPLEMENT ACTIVITY AMONG INBRED STRAINS OF MICE. J Immunol. 1964 Apr;92:576–578. [PubMed] [Google Scholar]
- Wiggins R. C., Giclas P. C., Henson P. M. Chemotactic activity generated from the fifth component of complement by plasma kallikrein of the rabbit. J Exp Med. 1981 Jun 1;153(6):1391–1404. doi: 10.1084/jem.153.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kessel K. P., Antonissen A. C., van Dijk H., Rademaker P. M., Willers J. M. Interactions of killed Listeria monocytogenes with the mouse complement system. Infect Immun. 1981 Oct;34(1):16–19. doi: 10.1128/iai.34.1.16-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


