Abstract
Mice of the I/St and A/Sn inbred strains display a severe and moderate course, respectively, of disease caused by Mycobacterium tuberculosis. Earlier, we showed that the response to mycobacterial antigens in I/St mice compared to that in A/Sn mice is shifted toward Th2-like reactivity and a higher proliferative activity and turnover of T cells. However, the physiologic basis for different expressions of tuberculosis severity in these mice remains largely unknown. Here, we extend our previous observations with evidence that I/St interstitial lung macrophages are defective in the ability to inhibit mycobacterial growth and to survive following in vitro infection with M. tuberculosis H37Rv. A unique feature of this phenotype is its exclusive expression in freshly isolated lung macrophages. The defect is not displayed in ex vivo macrophages obtained from the peritoneal cavity nor in macrophages developed in vitro from progenitors extracted from various organs, including the lung itself. In addition, we show that, in sharp contrast to peritoneal macrophages, the mycobactericidal capacity of lung macrophages is not elevated in the presence of exogenous gamma interferon. Our data suggest that the in vivo differentiation in a particular anatomical microenvironment determines the pattern of macrophage-mycobacterium interaction. Thus, caution should be exercised when conclusions based upon the results obtained in a particular in vitro system are generalized to the functions of all phagocytes during M. tuberculosis infection.
The identification of genes and their alleles that confer resistance versus susceptibility to tuberculosis (TB) provides deep insight into basic mechanisms of immunity and pathology. Of utmost importance is establishing genotyping-based approaches for identifying the 10% of individuals who are at high risk of progression to clinical TB after primary infection (8). Although there is substantial evidence for the role of genetic factors in human susceptibility to TB (13, 42, 57), the identification of human TB susceptibility genes has been complicated by polygenic control of the trait (7, 30, 45, 53) and inability to clearly delineate clinical phenotypes for stratified genetic analysis (5, 55). Unlike rare mutant alleles of the genes IFNγR and IL-12R that determine extremely high susceptibility to mycobacterial infections in humans (2, 16, 31, 43)—resembling that in mice bearing disrupted genes for several key cytokines (14, 15, 25, 26)—genes with modest effects rarely produce clear phenotypes. Due to a limited power to detect such genes and to choose “major” genes among numerous candidates, little is known concerning the human genetics of TB control. Variations in NRAMP1 (1, 6) and/or NRAMP1-linked loci on the human chromosome 2q35 segment (28), as well as the class II HLA genes (10, 27, 40), have been shown to be linked to or associated with susceptibility to mycobacterial infections in humans. However, the overall effect of NRAMP1 and HLA genes on TB infection is acknowledged to be weak, and other genes are certain to play a role in susceptibility to TB (7, 30, 38).
Animal models of TB have proven to be extremely valuable in elucidating the mechanisms of immunity to mycobacteria and genetic control of susceptibility and resistance (30, 38, 55). Several aspects of T-cell-macrophage interactions and cytokine networks underlying the balance between attempts by the host to eradicate infection and by mycobacteria to survive and multiply within the host are similar in humans and animals (20, 47, 51), making interspecies assumptions reasonable. Given the ∼85% homology between the mouse and human genomes (38, 55), susceptibility and resistance genes identified in murine models can be investigated as putative candidates in humans.
Using the approaches of whole-genome scanning and recombinant congenic strains in experimental mouse models, investigators (32, 33, 49) have genetically mapped variations in quantitative TB phenotypes, such as survival time, dynamics of body weight loss, and degree of lung pathology, following Mycobacterium tuberculosis challenge. Several nonlinked genetic loci appear to control these complex phenotypes. In our model involving mouse strains that display a severe (I/St), moderate (A/Sn), or mild [(I/St × A/Sn)F1] course of disease caused by M. tuberculosis (33, 44), microsatellite linkage analysis significantly or suggestively linked relevant quantitative trait loci (QTL) to distal Chr 3, proximal Chr 9, and mid-proximal Chr 17 (33, 49). Despite the fact that chromosome regions surrounding all of these QTL contain genes that regulate functions of cells of the immune system (candidate genes [33]), the physiologic basis for different expressions of TB severity in I/St and A/Sn mice remains unknown.
Assuming that this difference most likely depends upon peculiarities of immune responses in the lungs, i.e., the anatomical site where TB predominantly develops, we performed interstrain comparative immunologic analysis of cell-mediated responses against mycobacteria in the lung (35, 36). Significantly more activated CD8+ T cells accumulated in the lungs of resistant A/Sn and F1 mice than in those of I/St mice. In contrast, irrespective of the mycobacterial load in the organs, CD4+ T cells from susceptible I/St mice proliferated much more vigorously than T cells from A/Sn mice in response to mycobacterial sonicate, both in vitro and in vivo. Furthermore, a higher proportion of I/St than of A/Sn CD4+ T cells expressed the CD95 marker and underwent apoptosis. Following TB challenge, lung cells of I/St origin produced more interleukin 5 and, especially, interleukin 10, whereas their A/Sn and F1 counterparts produced greater amounts of gamma interferon (IFN-γ) (35, 36). This supports the role of type 1 cytokines, e.g., IFN-γ, in the development of adaptive protective immunity against mycobacterial infections (19, 52). However, it did not seem likely that a moderate bias toward Th2-like reactivity and a higher proliferative activity and turnover of T cells were sufficient to severely impair TB protection in I/St mice.
Here, we extend our previous observations with evidence that I/St interstitial lung macrophages are defective in the ability to inhibit mycobacterial growth and to survive following in vitro infection with M. tuberculosis H37Rv. A unique feature of this phenotype is its exclusive expression in freshly isolated lung macrophages. The defect is not displayed in ex vivo macrophages obtained from a distant anatomical site, i.e., the peritoneal cavity, nor in macrophages developed in vitro from progenitors extracted from various organs, including the lung itself. In addition, we show that, in sharp contrast to peritoneal macrophages, the mycobactericidal capacity of lung macrophages isolated from animals previously unexposed to mycobacteria is not elevated in the presence of exogenous IFN-γ.
MATERIALS AND METHODS
Animals.
Inbred mice of the strains I/StSnEgYCit (I/St) and A/JSnYCit (A/Sn) and their (A/Sn × I/St)F1 hybrids were bred and maintained under conventional conditions in the Animal Facilities of the Central Institute for Tuberculosis, Moscow, Russia, in accordance with guidelines from the Russian Ministry of Health no. 755, Office of Laboratory Animal Welfare Assurance A5502-01. Water and food was provided ad libitum. Female mice 2 to 4 months of age were used as the source of cells; cells from three or four mice of each strain were extracted in each experiment, and syngeneic cells were always mixed.
Media.
For adherence of macrophages to plastic, RPMI 1640 supplemented with 10% fetal calf serum (FCS), 10 mM HEPES, 2 mM l-glutamine, 1% nonessential amino acids, 1 mM pyruvate, 5 × 10−5 M 2-mercaptoethanol, and antibiotics (all components from HyClone, Carlington, The Netherlands) was used (medium 1). All assays were performed in a medium which differed from medium 1 in that it contained no antibiotics and only 2% FCS (medium 2). In preliminary titration experiments, it was shown that higher concentrations of FCS substantially suppressed extracellular mycobacterial growth. The inhibitory effect of FCS was readily abrogated by adding ferric ammonium citrate (Sigma, St. Louis, Mo.) to the medium (data not shown), suggesting that inhibition of mycobacterial growth at high FCS concentrations was due to transferrin-dependent consumption of iron. The viabilities and phagocytic capacities of macrophages were similar at 2 and 10% FCS contents (data not shown).
Mycobacteria.
M. tuberculosis strain H37Rv (Pasteur), a kind gift of G. Marchale (Intitute Pasteur, Paris, France), was maintained and prepared for macrophage in vitro infection exactly as described previously (35, 36, 44). Briefly, following 3 weeks of growth on Loewenstein-Jensen medium at 37°C, mycobacteria were suspended in sterile saline containing 0.05% Tween 20 and 0.1% bovine serum albumin (BSA; Sigma), washed, aliquoted (10 mg of semidry bacterial mass in 1 ml), and stored at −80°C. To obtain the log-phase bacteria for challenge, 50 μl from a thawed aliquot was added to 5 ml of Dubos broth (Difco, Detroit, Mich.) supplemented with 0.5% BSA and oleic acid and incubated for 1 week at 37°C. The mycobacterial suspension (0.5 ml) was diluted in 20 ml of fresh warm Dubos-BSA medium and further cultured for 1 week. The resulting suspension was washed three times at 3,000 × g and 4°C with 0.02% EDTA-phosphate-buffered saline (PBS) (Ca2+- and Mg2+-free) solution, resuspended in medium 2, and filtered through a 4-μm-pore-size filter (Sigma) to remove clumps. To estimate the CFU content in the filtrate, 10 μl from each fivefold serial dilution was plated onto Dubos agar (Difco), and the total number of microcolonies in the spot was calculated under an inverted CK-2 microscope (Olympus, Osaka, Japan) after being cultured for 3 days at 37°C. The bulk of the filtered culture was stored at 4°C, and it was found that no change in the CFU content occurred during this period.
Macrophage isolation. (i) Interstitial lung macrophages.
To obtain macrophages from the lungs, mice were euthanized by injection of an overdose of thiopental (Biochemie GmbH, Vienna, Austria), and lung cell suspensions were prepared using methods described earlier (35, 36). Briefly, the blood vessels were washed out, and repeated bronchoalveolar lavage was performed using 0.02% EDTA-PBS with antibiotics. The lung tissue was sliced into 1- to 2-mm3 pieces and incubated at 37°C for 90 min in RPMI 1640 containing 5% FCS, antibiotics, 10 mM HEPES (all components from HyClone), 200 U of collagenase/ml, and 50 U of DNase I (Sigma)/ml. Single-cell suspensions were obtained by vigorous pipetting. The lung cells were washed twice in Hanks' balanced salt solution (HBSS) containing 2% FCS and antibiotics and resuspended in medium 1. Cells (20 × 106 to 30 × 106) were incubated in 10 ml of medium 1 for 1.5 h on 90-mm-diameter petri dishes (Costar-Corning, Badhoevedorp, The Netherlands) at 37°C. Nonadherent cells were removed by triple vigorous washing with warm antibiotic-free HBSS containing 2% FCS. Adherent cells were detached from the plastic by incubating the monolayers in 0.02% EDTA-PBS antibiotic-free solution for 30 min at room temperature. Cell suspensions were obtained by pipetting, washed twice with HBSS, and resuspended in medium 2. The viability of lung macrophages, as determined by trypan blue exclusion, was >93%, and the content of nonspecific esterase-positive cells was >85%.
(ii) Peritoneal macrophages.
Mice were injected intraperitoneally with 3% peptone (Sigma) in saline. Five days later, peritoneal exudate cells were eluted from the peritoneal cavities with HBSS supplemented with 2% FCS and 10 U of heparin/ml, washed twice, and resuspended in medium 1. The subsequent procedures were identical to those described above for the lung macrophages. The viability and purity of peritoneal macrophages exceeded 95%.
(iii) Development of macrophages in vitro in the presence of L929 supernatant.
Spleens were homogenized and lungs were enzymatically disrupted as described above. After 2 h of adherence to plastic at 37°C, nonadherent cells were removed and the cells were cultured in medium 1 containing 20% supernatant from a 1-week-old culture of the L929 cell line in medium 1. After 10 to 14 days of culture, the cells were detached and resuspended in medium 2 as described above. Bone marrow cells were obtained from femurs of mice and cultured for 3 days in medium 1 containing 200 U of recombinant granulocyte-macrophage colony-stimulating factor (PeproTech, Rocky Hill, N.J.)/ml. Nonadherent cells were completely removed. Adherent cells were further cultured for 10 days in medium 1 containing 20% supernatant from a 1-week-old culture of L929 cells, detached, and resuspended in medium 2. The viabilities and purities of all types of in vitro-developed macrophages exceeded 95%.
Antimycobacterial activities of macrophages.
To assess the basic parameters of phagocytosis (Table 1), mycobacteria were added to lung macrophage cultures at multiplicities of infection (MOIs) of 2 and 8. After 6 h of incubation, the monolayers were fixed with 2% paraformaldehyde and stained for mycobacteria with auramine O (Sigma) with Giemsa counterstaining, as recommended by the manufacturer. The bottom of each well was removed using a hollow steel punch, and the disk was attached to a microscope slide with nonfluorescent viscous oil. The basic parameters of phagocytosis were estimated under epifluorescent illumination using an Olympus BH-2 microscope (magnification, ×1,250). A total of 400 cells in at least 35 fields per point were assessed in two independent experiments. No bacilli unassociated with macrophages were found during the first 18 h of culture.
TABLE 1.
Parameters of mycobacterial phagocytosis do not differ in I/St and A/Sn lung macrophage culturesa
| Mouse strain | % Nonspecific esterase-positive cells | % Phagocytosisb
|
Phagocytic numberc
|
||
|---|---|---|---|---|---|
| 2:1d | 8:1 | 2:1 | 8:1 | ||
| I/St | 88 ± 7 | 27 ± 5 | 38 ± 7 | 105 ± 15 | 181 ± 16 |
| A/Sn | 86 ± 8 | 29 ± 5 | 46 ± 8 | 88 ± 13 | 154 ± 23 |
Plastic-adherent interstitial lung cells were prepared as described in Materials and Methods and plated at 3 × 105/well in 24-well tissue culture plates (Costar-Corning). Single-cell mycobacterial suspensions were added at the indicated MOIs. After 6 h of incubation, the monolayers were fixed with 2% paraformaldehyde and stained with auromine O with Giemsa counterstaining. The basic parameters of phagocytosis were estimated microscopically (magnification, × 1,500). A total of 400 cells in at least 35 fields per point were assessed in two independent experiments. No significant interstrain differences were observed (P > 0.05).
Percentage of cells containing at least one mycobacterium.
Total number of mycobacteria per 100 phagocytic cells.
MOI.
To assess the antimycobacterial activities of macrophages from different sources, we used methods described earlier (35, 36). Briefly, 2 × 104 (in vitro-developed), 4 × 104 (lung), or 5 × 104 (peritoneal) macrophages were plated in a well of a flat-bottom 96-well plate (Costar-Corning) to obtain monolayers of good density. The cells were allowed to adhere for 1 h before live mycobacteria were added at the MOIs indicated in the figures and tables. The growth of mycobacteria was assessed by [3H]uracil uptake exactly as described previously (32-37). Briefly, 1 μCi of [3H]uracil (Isotop, St. Petersburg, Russia)/well was added for the last 18 h of 60- to 108-h mycobacterium-macrophage cocultures and to the control wells containing mycobacteria alone. The cultures were terminated by freezing the contents of the wells at −30°C and were harvested on fiberglass filters (Scatron, Oslo, Norway) after the contents were thawed. [3H]uracil uptake by mycobacteria was measured in a liquid scintillation counter (Wallac, Turku, Finland). The results are expressed as mean counts per minute ± standard deviation (SD) for triplicate cultures. To evaluate how precisely this surrogate readout corresponds to actual numbers of live mycobacteria in cocultures, specific comparative experiments were undertaken. As shown in Fig. 1, parallel estimations of mycobacterial CFU counts and [3H]uracil uptake under identical culture conditions and dynamics provided coefficients of correlation of >0.9, validating the use of the latter, less laborious and variable technique throughout the experiments.
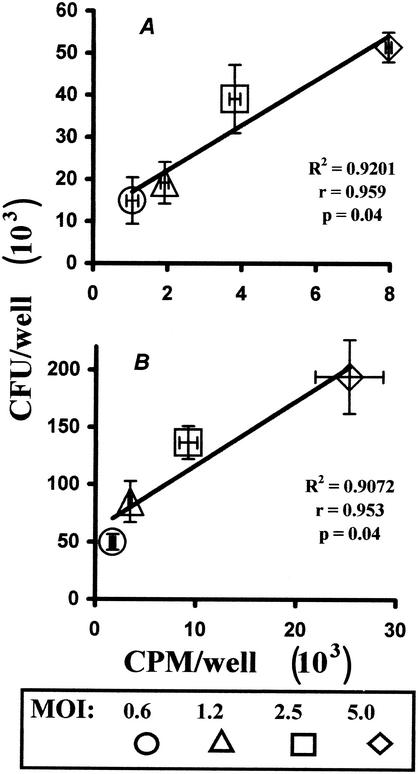
FIG. 1.
Assessment of mycobacterial content in in vitro-infected macrophage cultures by CFU counting and [3H]uracil uptake provides consistent results. Peritoneal macrophages were isolated from (I/St × A/Sn)F1 mice, plated, and infected at various MOIs as described in Materials and Methods. After 24 (A) or 48 (B) h of incubation, the cultures were either pulsed with 1 μCi of [3H]uracil/well for the last 18 h of incubation or lysed by replacement of the culture medium with Dubos broth containing 0.08% sodium dodecyl sulfate (SDS). After 10 min of lysis, 20% BSA (Sigma) in sterile water was added to neutralize the SDS, and the contents of the wells were plated on Dubos agar medium. Mycobacterial CFU were counted after 16 days of incubation at 37°C. The CFU counts were plotted against counts per minute (CPM) obtained in identically infected cultures; the error bars represent SDs for these two parameters.
Macrophage viability was determined at different times after they were loaded with M. tuberculosis by counting the number of cells that remained adherent and retained the appearance of uninfected control macrophages obtained from the same source. During microscopic examination, the difference between live and dead macrophages was readily seen without the use of vital dyes, as illustrated in Fig. 2. Live- and total cell counts (Fig. 3) were obtained using an inverted CK-2 phase microscope equipped with a 10-mm2 grid in the eyepiece. Three random areas per well and three wells per condition were counted at ×100 magnification, as described previously (46).
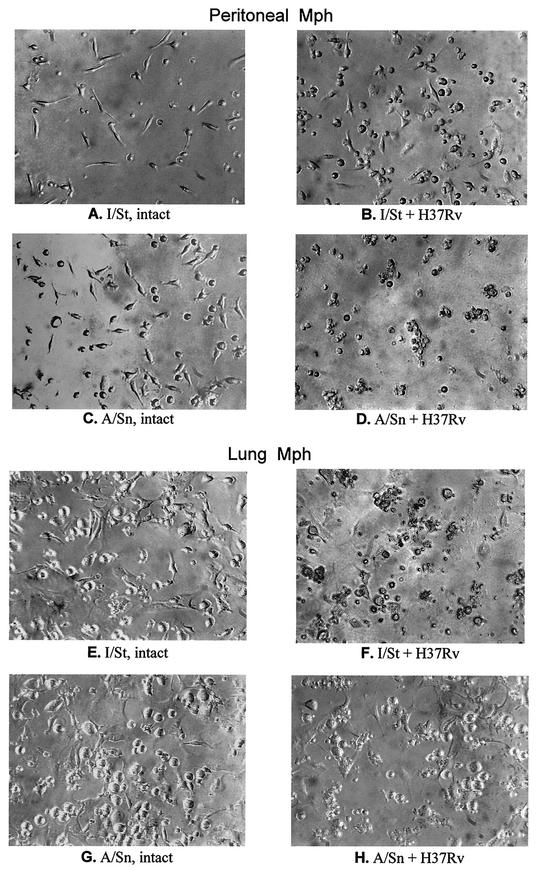
FIG. 2.
Cytopathic effect of M. tuberculosis H37Rv in I/St and A/Sn peritoneal and lung macrophages infected in vitro. Macrophages (Mph) from the peritoneal cavity (A to D) and from interstitial lung tissue (E to H) were isolated, purified, and plated as described in Materials and Methods. The cells were either left uninfected (A, C, E, and G), or mid-log-phase H37Rv clump-free bacilli were added (B, D, F, and H). Analysis at various MOIs and culture duration intervals always provided consistent results. The micrographs were taken with an inverted microscope (magnification, ×190) during one representative experiment out of eight similar experiments and reflect the situation after 72 h of culture (initial MOI = 3 for infected cells).
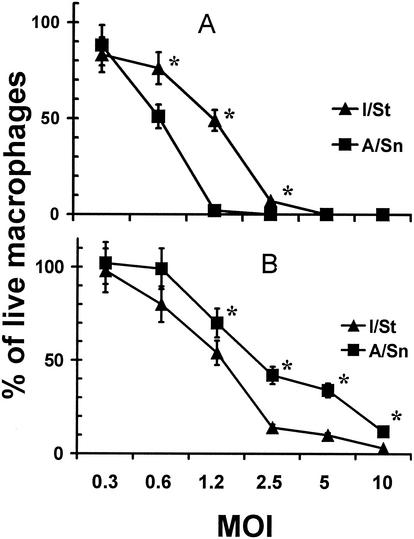
FIG. 3.
Interstrain differences in viability of I/St and A/Sn peritoneal and lung macrophages following in vitro infection with M. tuberculosis H37Rv. Peritoneal (A) and lung (B) macrophages were isolated, purified, plated, and infected (see Materials and Methods). The number of cells that remained adherent and retained the appearance of uninfected control macrophages from the same source, as well as the total number of cells, were determined using an inverted microscope equipped with a 10-mm2 grid in the eyepiece. Three random areas per well and three wells per condition were counted at ×100 magnification with estimation of the mean. The results obtained in four independent experiments at 72 h of culture are displayed as an aggregate mean of the percentage of live cells ± SD. Interstrain differences were statistically significant (P < 0.01 to 0.05; Mann-Whitney U test) at MOIs of 0.6 to 2.5 for peritoneal macrophages and 1.2 to 10.0 for lung macrophages (marked with asterisks). Similar results, although with statistically significant differences shifted to higher MOI values (2.5 to 10.0), as expected, were obtained in 48-h cultures.
Nitric oxide production.
Nitric oxide production was assessed in 36-h culture supernatants by measuring nitrite, a stable metabolite of NO, using the Griess reaction.
LDH release from macrophages.
Lactate dehydrogenase (LDH) release from macrophages, as a measure of macrophage lysis, was determined from the enzymatic activity of LDH in culture supernatants using a CytoTox 96 kit (Promega, Madison, Wis.) as recommended by the manufacturer. Per cent specific lysis was calculated according to the following formula: (A490 in experimental wells − A490 after spontaneous release)/(A490 after total lysis − A490 after spontaneous release) × 100.
TNF-α quantitation.
TNF-α was measured in 36-h culture supernatants with an enzyme-linked immunosorbent assay (ELISA) kit (PharMingen, San Diego, Calif.) with clone MP6-XT22 and polyclonal antibodies as the capture and detecting antibodies, respectively (sensitivity, 125 pg/ml). ELISAs were performed following the manufacturer's instructions.
Statistical analysis.
The significance of differences was estimated by nonparametric Wilcoxon and Mann-Whitney tests. A P value of <0.05 was considered statistically significant. Approximation (R2) and correlation (r) coefficients for the results depicted in Fig. 1 were obtained from Excel equations.
RESULTS
Mycobacterial growth in freshly isolated I/St and A/Sn macrophages.
To determine if virulent mycobacteria interact differently with macrophages from mice that differ with respect to the severity of TB, we used a macrophage-mycobacterial coculture model (see Materials and Methods) (35, 36). First, it was important to find out whether lung macrophages from I/St and A/Sn mice differ in the basic parameters of mycobacterial phagocytosis, since uneven starting conditions of mycobacterium-macrophage interaction in cultures could well account for all other phenotypic differences. As shown in Table 1, during the first 6 h of the experiment, no significant differences between lung macrophages of I/St and and A/Sn origin were observed in esterase-positive cell content and phagocytic capacity, indicating that the interstrain differences described below arose no earlier than at the stage of mycobacterial replication within macrophages.
In the first series of experiments, we studied the cytopathic action of M. tuberculosis H37Rv in I/St and A/Sn peritoneal and lung macrophages and the dynamics of mycobacterial growth in these cells. Microscopic examination of macrophage-mycobacterial cocultures demonstrated that I/St and A/Sn macrophages differ profoundly in the capacity to survive infection. As shown in Fig. 2, in the absence of mycobacteria, I/St and A/Sn macrophages looked very much alike on day 3 of the experiment (Fig. 2A and C for peritoneal macrophages and E and G for lung macrophages). Mycobacterial infection had a marked cytopathic effect upon macrophages, and this effect clearly differed between mouse strains. Paradoxically, whereas a good proportion of peritoneal macrophages from the more susceptible I/St mice survived and looked normal on day 3 of infection (Fig. 2B), no live A/Sn peritoneal macrophages remained in the culture (Fig. 2D). In sharp contrast, I/St lung macrophages were completely destroyed at an MOI bearable by their A/Sn counterparts (Fig. 2F and H, respectively).
These interstrain differences were dynamic and quantitative: higher MOI and/or prolongation of the coculture period resulted eventually in the killing of all macrophages. However, the differences were readily observed within a wide variety of infective doses. As shown in Fig. 3, the percentage of live macrophages in culture wells, as estimated with an inverted phase microscope equipped with a grid in the eyepiece, was inversely related to the MOI, and the cells retained strain-specific and tissue-specific patterns of survival. Moreover, the pattern of interstrain differences remained unaltered for >1 week of culture at an MOI of <1 (data not shown).
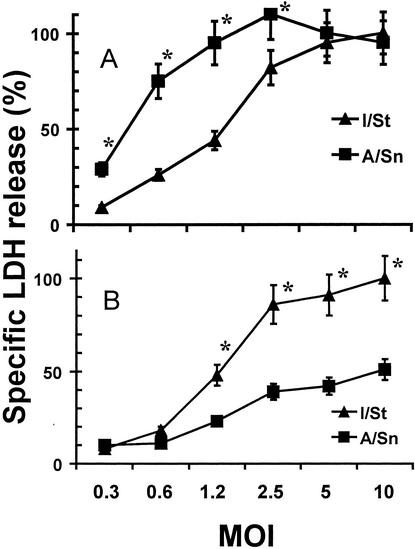
In order to assess the cytopathic effect of mycobacteria on macrophages in a more quantitative and objective manner, we measured macrophage disintegration by a common method of LDH release, taking advantage of the lack of LDH in mycobacterial cells. Figure 4 shows that the pattern of LDH release from infected cells was in full agreement with microscopic data: I/St lung macrophages were more sensitive, while peritoneal macrophages were less sensitive, to the cytopathic action of mycobacteria.
FIG. 4.
LDH release from infected I/St and A/Sn macrophages. The deaths of peritoneal (A) and lung (B) macrophages were assessed by specific LDH release into culture supernatants using a Promega CytoTox 96 kit. The percent specific cytotoxicity was calculated as described in Materials and Methods. The results are means ± SDs from three independent 72-h experiments. Statistically significant (P < 0.01 to 0.001; Wilcoxon test) interstrain differences are marked with asterisks. Very similar results were obtained in 48-h experiments.
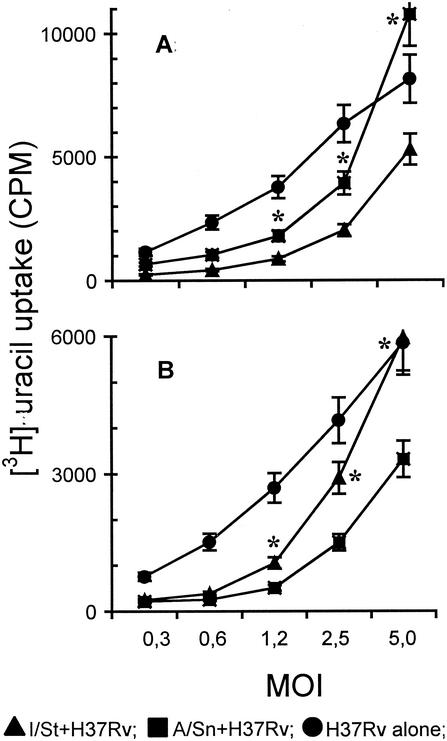
To discover whether the rates of mycobacterial growth in I/St and A/Sn macrophages are different, we measured the level of [3H]uracil uptake by mycobacteria, a parameter which correlates well with mycobacterial multiplication (3, 56). As shown in Fig. 5, mycobacterial growth in macrophages of either strain was reduced compared to that in macrophage-free cultures. In agreement with cytotoxicity data, the bacteriostatic capacity of peritoneal macrophages from more susceptible I/St mice was significantly higher than that of their A/Sn counterparts (Fig. 5A), whereas lung macrophages followed the pattern of genetically determined susceptibility to TB in the corresponding mouse strains. At a 60-h culture interval, the speed of mycobacterial growth in I/St lung macrophages exceeded that in A/Sn macrophages by ∼2-fold (Fig. 5B). Analogous results were obtained in 2- and 5-day cultures (data not shown).
FIG. 5.
Mycobacterial growth in I/St and A/Sn macrophages following in vitro infection. Peritoneal (A) and lung (B) macrophages were prepared, plated, and infected as described above. The rate of mycobacterial growth was measured by [3H]uracil uptake at different time points after cocultures were established. [3H]uracil (1 μCi/well) was added for the last 18 h of incubation. The wells containing mycobacteria alone at numbers corresponding to each MOI served as controls. The results obtained in one of eight similar 60-h experiments are expressed as mean counts per minute (CPM) ± SD for triplicate cultures, and statistically significant (P < 0.01; Mann-Whitney U test) interstrain differences are marked with asterisks. Consistent results were obtained in 84- and 108-h experiments (data not shown).
Influence of exogenous IFN-γ on NO production and mycobacterial growth inhibition in macrophage cultures.
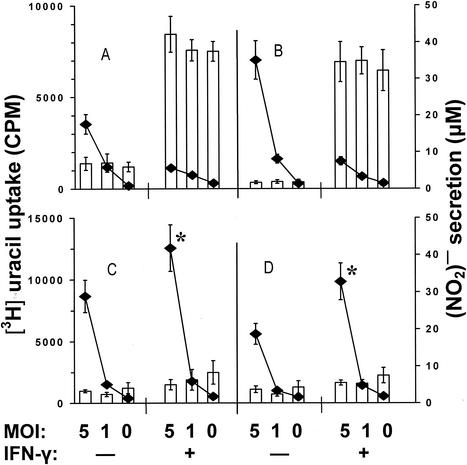
There is ample evidence that one of the main mechanisms of macrophage activation for mycobacterial killing in mice is IFN-γ-mediated induction of reactive nitrogen intermediates (12, 23, 37). However, it remains unclear whether pulmonary macrophages respond to activation by IFN-γ in the same way (4; G. Zissel, D. V. Pechkovsky, C. Stamme, M. Einhaus, C. Taube, H. Magnussen, M. Schlaak, and J. Muller-Quernheim, Abstr. 11th Int. Congr. Immun., abstr. A6. Tue.4. 17/766, 2001) and whether IFN-γ alone is sufficient to stimulate a mycobacteriostatic response (9). To address these issues, we cocultured mycobacteria with peritoneal and lung macrophages from the two mouse strains in the presence or absence of 100 U of murine recombinant IFN-γ (rIFN-γ)/ml (Genzyme, Cambridge, Mass.) and assessed NO production and mycobacterial multiplication in the corresponding cultures (Fig. 6). I/St peritoneal macrophages, both infected with mycobacteria and uninfected, produced small but measurable amounts of NO without stimulation with IFN-γ (Fig. 6A). In contrast, their A/Sn counterparts did not produce NO under these conditions (Fig. 6B). In agreement with previous findings, [3H]uracil uptake by mycobacteria was higher in A/Sn cultures. This suggests that the interstrain difference in the bacteriostatic capacities of peritoneal macrophages could be due to an intrinsic difference in the quantities of NO produced. However, the shapes of titration curves (Fig. 6), which are in sharp contrast with monotonous NO production within the corresponding experimental groups, indicate that the level of [3H]uracil incorporation depended exclusively upon the initial MOI and that even in I/St cultures, NO concentrations did not reach the threshold required for significant mycobacterial growth inhibition. Culturing of peritoneal macrophages of either mouse strain in the presence of exogenous rIFN-γ resulted in a prominent increase in NO production. High NO levels were not diminished when macrophages were infected with mycobacteria and were sufficient to markedly and equally reduce mycobacterial multiplication in I/St and A/Sn cultures (Fig. 6A and B).
FIG. 6.
Influence of exogenous IFN-γ on macrophage NO production (bars) and mycobacterial growth inhibition (curves). Peritoneal (A and B) and lung (C and D) macrophages from I/St (A and C) and A/Sn (B and D) mice were prepared, plated, and infected as described above. Macrophage-mycobacterial cocultures were incubated in the presence (+) or absence (−) of 100 U of murine rIFN-γ/ml for 60 h in triplicate. Some cultures were pulsed with [3H]uracil for the last 18 h to assess mycobacterial growth. The nitric oxide contents in the culture supernatants were measured by Griess reaction. The results are means ± SDs for triplicates from one of two similar experiments. The addition of IFN-γ to I/St and A/Sn peritoneal macrophages (A and B) significantly increased NO production (P < 0.001; Mann-Whitney U test) and inhibited mycobacterial growth (P < 0.01). In lung macrophages (C and D) at an MOI of 5, mycobacteria multiplied more vigorously when IFN-γ was added to the cultures (asterisks, P < 0.05; Mann-Whitney U test). CPM, counts per minute.
Interstitial lung macrophages differed dramatically from peritoneal macrophages with respect to the phenotypes under study (Fig. 6C and D). First, in the absence of IFN-γ, the levels of NO production were marginal in both I/St and A/Sn cultures. Second, NO production by lung macrophages did not increase significantly in the presence of exogenous IFN-γ. Third, at high MOIs (Fig. 6C and D show an MOI of 5; similar results were obtained in short-term cultures for MOIs of 10 and 20 [data not shown]), mycobacterial multiplication was not lower but higher (P < 0.05) when IFN-γ was added to either I/St or A/Sn cultures.
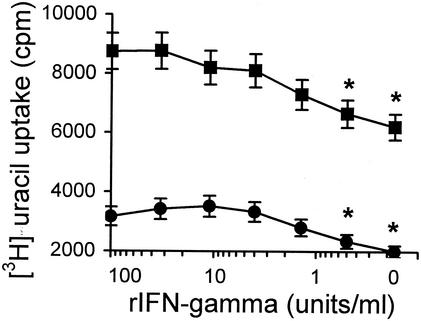
Given that mycobacteria multiply in macrophage-free cultures more vigorously than in the presence of macrophages (Fig. 5), and taking into account that under microscopic examination mycobacterium-infected lung macrophages looked less healthy in the presence of IFN-γ than in its absence (data not shown), we investigated the possibility that elevated mycobacterial growth was due to impaired survival of lung macrophages in the presence of exogenous IFN-γ. To test this hypothesis directly, we assessed [3H]uracil uptake by mycobacteria in infected I/St lung macrophage cultures in the presence of diminishing amounts of IFN-γ. Cytokine titration curves (Fig. 7) demonstrate that mycobacteria grow better if exogenous IFN-γ is added to cocultures, even in small amounts.
FIG. 7.
Mycobacteria multiply better in lung macrophages in the presence of exogenous IFN-γ. Mycobacteria were added to lung macrophages at an MOI of 2 (circles) or 5 (squares), and the cocultures were incubated for 60 h (with [3H]uracil for the last 18 h) in the presence of serial threefold dilutions of IFN-γ as indicated on the x axis. The results are shown as mean counts per minute (cpm) ± SD for triplicate cultures in one of two similar experiments. At IFN-γ concentrations of <1 U/ml, [3H]uracil uptake was significantly lower (asterisks, P < 0.05; Mann-Whitney U test) than at concentrations between 3 and 100 U/ml.
In T-cell-free macrophage cultures, TNF-α is the cytokine most likely to activate macrophages and influence mycobacterial growth. To find out if I/St and A/Sn lung macrophages differ in TNF-α production, we measured the TNF-α content in culture supernatants under different experimental conditions. As shown in Table 2, compared to cells of I/St origin, A/Sn lung macrophages produced about twice as much TNF-α (P < 0.05; Mann-Whitney U test). Adding either rIFN-γ or mycobacterial sonicate to the cultures increased the amount of TNF-α ∼2-fold, while mycobacterial infection slightly diminished TNF-α production (probably due to a cytopathic effect). However, a significant (P < 0.05) twofold interstrain difference remained constant under all these conditions. Thus, an intrinsic difference between I/St and A/Sn lung macrophages in the ability to produce TNF-α could be one of the reasons for their different abilities to combat mycobacteria.
TABLE 2.
TNF-α production by I/St and A/Sn lung macrophagesa
| Mouse strain | IFN-γ in culture | TNF-α production after loading of macrophages
|
|||
|---|---|---|---|---|---|
| None (control) | H37Rv (2:1) | H37Rv (1:1) | H37Rv sonicateb | ||
| I/St | − | 1,440 ± 170 | 1,335 ± 155 | 1,200 ± 105 | 1,990 ± 250 |
| + | 2,200 ± 210 | 1,995 ± 180 | 2,370 ± 210 | ND | |
| A/Sn | − | 3,105 ± 345 | 2,260 ± 225 | 2,505 ± 190 | 5,900 ± 315 |
| + | 4,900 ± 310 | 4,100 ± 320 | 4,710 ± 385 | ND | |
Lung macrophages (5 × 104) obtained as described in Materials and Methods were cultured in the wells of a 96-well flat-bottom plate (Costar) for 24 h in the presence (+) or absence (−) of the indicated stimuli. The TNF-α contents in undiluted and 1:1-diluted culture supernatants were measured by ELISA in triplicate. The results were plotted against the curve experimentally obtained by serial dilutions of recombinant TNF-α (PharMingen) and are expressed in picograms per milliliter ± SD for the aggregate mean of the measurements (six wells per point). The results of one of two similar experiments are displayed.
A mixture of H37Rv-derived soluble substances was obtained by means of ultrasonication as described elsewhere (44) and used as the antigen at 10 μg/ml. ND, not determined.
Inheritance of antimycobacterial macrophage response and unique phenotype of lung macrophages.
It has been shown that following infection with M. tuberculosis given intravenously, i.e., by a systemic route, (I/St × A/Sn)F1 hybrids survived significantly longer than mice of either parental strain, thus expressing a hyperresistant phenotype (33, 35). The dichotomy in antimycobacterial responses between pulmonary and extrapulmonary macrophages (see above) suggested that heterozygous F1 hybrids combat systemic mycobacterial infection better than homozygous parental mice because their macrophages from different anatomic locations express favorable phenotypes of both parental strains. In other words, F1 hybrids could inherit in a dominant manner a better antimycobacterial response of lung and nonlung macrophages from A/Sn and I/St mice, respectively.
To address this question, we compared mycobacterial multiplication and macrophage LDH release in lung and peritoneal macrophages of the two parental strains and their F1 hybrids. As shown in Table 3, F1 hybrids demonstrated intermediate phenotypes with respect to both criteria. Thus, superdominance, or a heterosis effect, observed earlier in mortality experiments was not due to complementation between antimycobacterial responses provided by macrophages from distinct anatomical sites.
TABLE 3.
Macrophages from (I/St × A/Sn)F1 hybrids demonstrate intermediate antimycobacterial response compared to parental-strain macrophagesa
| Macrophage origin | Parameterb | MOI | Value for mouse strain
|
||
|---|---|---|---|---|---|
| I/St | A/Sn | (I/St × A/Sn)F1c | |||
| Peritoneal | Specific LDH release (%) | 10 | 86 ± 7 | 89 ± 7 | ND |
| 5 | 63 ± 4 | 90 ± 8 | ND | ||
| 2.5 | 24 ± 1 | 55 ± 4 | ND | ||
| [3H]uracil uptake (cpm) | 10 | 10,100 ± 984 | 16,119 ± 905 | 14,373 ± 1,008 | |
| 5 | 4,177 ± 507 | 7,478 ± 674 | 6,013 ± 617 | ||
| 2.5 | 1,578 ± 312 | 3,076 ± 298 | 2,466 ± 196 | ||
| Lung | Specific LDH release (%) | 10 | 82 ± 5 | 58 ± 4 | 66 ± 6 |
| 5 | 75 ± 5 | 43 ± 3 | 53 ± 4 | ||
| 2.5 | 53 ± 5 | 31 ± 2 | 44 ± 5 | ||
| [3H]uracil uptake (cpm) | 10 | 26,876 ± 2,225 | 11,920 ± 997 | 20,676 ± 1,766 | |
| 5 | 8,458 ± 756 | 3,898 ± 406 | 6,169 ± 558 | ||
| 2.5 | 1,961 ± 188 | 1,652 ± 132 | 1,588 ± 167 | ||
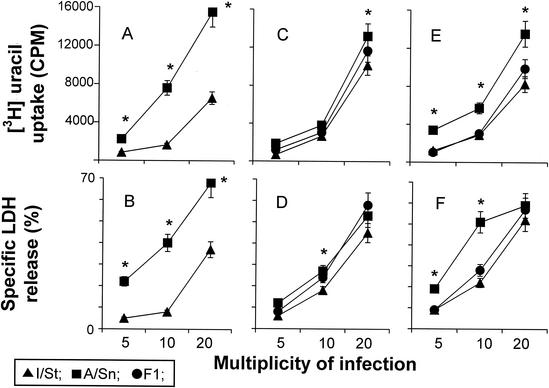
Nevertheless, since peritoneal macrophages are not the cells that predominantly interact with mycobacteria during TB infection, the possibility remained that phagocytes residing in other organs and tissues are responsible for the complementation effect. In addition, much of the previous data on mycobacterium-macrophage interactions were obtained in coculture systems utilizing macrophage-derived cell lines, macrophages developed from peripheral blood mononuclear cells in long-term in vitro cultures, or macrophages developed in vitro in the presence of growth and differentiation factors (see references 24 and 51 for reviews). Thus, it was important to find out if macrophages grown in vitro from progenitors obtained from different sources interacted with mycobacteria in a manner which was consistent with the genetics of the host. As shown in Fig. 8, macrophages developed in vitro from plastic-adherent bone marrow cells resembled peritoneal macrophages in that the ability to inhibit mycobacterial infection was higher (P < 0.01) in cells derived from TB-susceptible I/St mice (Fig. 8A and B) and that IFN-γ increased the antimycobacterial response (data not shown). Interstrain differences between macrophages derived from spleen- and lung-residing progenitors were also significant (P < 0.05), although less pronounced, but again showed an inverse pattern of antimycobacterial response compared to the ex vivo lung macrophages (Fig. 8C and F). In all cases, an intermediate, not superdominant, type of inheritance was observed. Importantly, even macrophages derived from plastic-adherent interstitial lung cells, accumulated under artificial culture conditions, did not express the “genetically correct” phenotype that was characteristic of their counterparts naturally differentiated in and freshly isolated from the same tissue.
FIG. 8.
Mycobacterial growth in and cytopathic effect on macrophages developed in vitro. Macrophages were developed from progenitors residing in bone marrow (A and B), spleen (C and D), and lungs (E and F) by culturing them for 10 to 14 days in the presence of growth factors as described in Materials and Methods; they were then detached and put into the wells of flat-bottom 96-well plates at 20 × 103/well. The cells were infected with M. tuberculosis at the indicated MOIs, and [3H]uracil uptake (A, C, and E) and LDH release (B, D, and F) in 72-h cultures were measured as described above. The results are shown as means ± SDs for triplicate cultures in one of two similar experiments. In all cases, mycobacteria multiplied more vigorously and killed the host cells more readily in A/Sn than in I/St macrophages. Significant interstrain differences (P < 0.001 to 0.05; Mann-Whitney U test) are marked with asterisks. In all cases, an intermediate type of inheritance by F1 macrophages of the phenotypes under study was observed.
DISCUSSION
Studies of genetic control of infectious diseases in humans rely on clearly defined clinical phenotypes of the disease, a definition that is often arbitrary and not always possible. Thus, mapping and cloning of resistance or susceptibility genes in murine models of the disease, followed by comparative genome analysis, are extremely useful to identify their human homologues (34), and this approach is fully applicable to TB. However, as outlined earlier (30, 33, 55), the success of genetic analysis in animals also depends upon the identification of clear, extreme phenotypes of susceptibility to, or manifestation of, the disease. Since there is no definitive proof that any of the assessable phenotypes that characterize M. tuberculosis-induced disease in mice is the best correlate of TB susceptibility and severity in humans, current genetic studies are based on models that employ mouse strains which clearly differ in major complex phenotypic traits, such as mean survival time, wasting, lung pathology, or CFU counts in internal organs (32, 33). While they are advantageous for genetic analysis, these complex phenotypes pose a particular problem: their origins are difficult to trace back to primary cellular and genetic mechanisms.
In the present study, we describe a TB phenotype with the following characteristics: (i) it is readily measurable in a simple in vitro culture model that includes only two components—freshly isolated ex vivo host cells of a single type and virulent mycobacteria; (ii) it strictly follows the pattern of interstrain differences in TB severity as described for the corresponding mouse strains at the whole-animal level; and (iii) its expression is limited to the type of cells generally considered to be a central element of mycobacterium-host interaction, namely, pulmonary macrophages.
Pulmonary macrophages apparently have a dual role in M. tuberculosis infection. As a niche supporting the intracellular growth of M. tuberculosis (21, 41), they are important in the initial containment of mycobacteria through innate resistance mechanisms (58), and they are involved, both as antigen-presenting cells and as potential targets for cytotoxic T lymphocytes, in adaptive immunity to TB (51). To successfully serve the second set of protective functions, it is important for macrophages to resist the cytotoxic action of mycobacteria. As shown in Fig. 2 to 4, lung macrophages from A/Sn mice were more resistant to the cytopathic effect of mycobacteria than their I/St counterparts. These results are in full agreement with genetic data (33, 49) and may explain why mice of the former strain display a longer survival time and a less severe lung pathology (44). Of particular importance is the fact that better lung macrophage survival correlates with stronger inhibition of mycobacterial growth in cultures (Fig. 5). Dobos et al. (18) showed recently that lung epithelial cells of human origin, the A549 cell line, undergo necrosis following infection in vitro with virulent M. tuberculosis, which is in agreement with our results obtained in macrophage cultures. However, contrary to our results, in their experiments there was no inverse correlation between cell necrosis and the level of mycobacterial multiplication. Numerous factors could underlie this discrepancy, including different mechanisms of mycobacterial binding, invasion, and phagosome formation in the two cell types (39). Whatever the reason, these data suggest that, although several types of lung cells may be destroyed during mycobacterial growth, prolonged survival of professional phagocytes in TB lesions is probably the most important factor for restricting mycobacterial multiplication.
Another important issue that arises from the present study concerns the accuracy of extrapolation of the data obtained in experiments utilizing many different macrophage sources to mycobacterium-macrophage interactions in the lungs (or other affected organs). Starting from early studies of the role of IFN-γ in antimycobacterial response (22), many characteristics of macrophage-mycobacterium interaction were obtained in cultural systems utilizing phagocytic cells from different origins, e.g., immortalized cell lines and human peripheral blood mononuclear cells (see references 3, 21, 50, and 29 for examples and references 20, 47, and 51 for reviews). Our data, as shown in Fig. 6 and 8, indicate that at least genetic control of TB susceptibility at the macrophage level strongly depends upon the anatomical origin of the phagocytic cells used in the study. With respect to the TB phenotype in A/Sn and I/St macrophages, not only spleen- and bone marrow-derived macrophages, but even macrophages of lung origin grown in vitro in a cocktail of growth factors (Fig. 8), differed profoundly from freshly isolated interstitial lung macrophages (Fig. 2 to 5).
To prove that unique features and interstrain differences in the antimycobacterial responses of lung macrophages observed in the in vitro system are expressed in vivo, experiments with long-living radiation bone marrow F1→I/St chimeras are under way in our laboratory. However, given that freshly isolated ex vivo peritoneal macrophages also behaved differently than lung macrophages, even now one can speculate that it is in vivo differentiation in a particular anatomical microenvironment that determines the pattern of macrophage-mycobacterium interaction. This, in turn, highlights two important issues. First, caution should be exercised when conclusions based upon the results obtained in a particular in vitro system are generalized to the functions of all phagocytes during M. tuberculosis infection. Second, when applying the material obtained in simplified experimental systems to gene expression studies (e.g., using DNA microarrays, which in principal is very convenient and far less complicated for interpretation than using ex vivo extracted tissues), the right choice of cells for culturing may be critical.
How important these considerations are is underlined by the study of the activation of macrophage antimycobacterial function by IFN-γ. As shown in Fig. 6 and 7, the addition of exogenous IFN-γ resulted in deleterious, rather than protective, effects in cultures of lung macrophages. In contrast, peritoneal and bone marrow-derived phagocytes were readily activated by IFN-γ for mycobacteriostatic activity and protected themselves against mycobacterium-mediated cytopathic action. Aston et al. (4) demonstrated profound interspecies differences in a comparison of human alveolar macrophages and mouse peritoneal macrophages with regard to the ability to inhibit the growth of nonvirulent mycobacteria by producing nitric oxide in response to IFN-γ. However, our data indicate that the tissue specificity of macrophages is probably equally important, at least in the case of infection with virulent mycobacteria. A hypothesis that a second signal is needed in addition to IFN-γ for optimal macrophage activation has received substantial support, and a number of different candidates for these signals have been proposed (11, 17, 48, 54). There could be specific requirements for such a signal for interstitial lung macrophages that were not met under our culture conditions. In this regard, a very recent study (Zissel et al., Abstr. 11th Intern. Congr. Immun.) of the dependence of IFN-γ activation of alveolar macrophages on lung epithelial cells (which were not present in our cultures due to a highly selective method of macrophage purification) deserves special attention.
Finally, there is an interesting genetic aspect of the results reported here. F1 hybrids between the two mouse strains with different TB severities display a hyperresistant phenotype at the whole-animal level (33, 35); however, their macrophages, including those from the lung, express an intermediate level of TB defense (Table 3 and Fig. 8). It was shown very recently (49) that two out of three QTL that definitely participate in the genetic control of TB in our model, namely, tbs1 and tbs2, are active predominantly in female mice and provide an advantage to hetorozygous carriers. Both characteristics clearly differ from what is seen in a macrophage model, in which heterozygous F1 mice express an intermediate phenotype. On the other hand, a QTL linked to the H-2 complex on Chr 17 and thus to intra-MHC loci encoding TNF family molecules, is characterized by an intermediate type of TB severity inheritance. Although epistatic interactions demonstrated for all three QTL may interfere with straightforward analysis, linkage experiments are under way to determine if the unique phenotype of lung macrophages during interaction with virulent mycobacteria is controlled by an MHC-linked gene(s).
Acknowledgments
This work was supported by HHMI grant 753-01564101 (to A.S.A. as a Howard Hughes International Research Scholar) and Public Health Service grant RO1 HL68532-01 from the National Heart, Lung, and Blood Institute.
We thank D. McMurray for critically reading the manuscript.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abel, L., F. O. Sanchez, J. Oberti, N. V. Thuc, L. V. Hoa, V. D. Lap, E. Skamene, P. H. Lagrange, and E. Schurr. 1998. Susceptibility to leprosy is linked to the human NRAMP1 gene. J. Infect. Dis. 177:133-145. [DOI] [PubMed] [Google Scholar]
- 2.Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Doffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432-1435. [DOI] [PubMed] [Google Scholar]
- 3.Arias, M., M. Rojas, J. Zabaleta, J. I. Rodriguez, S. C. Paris, L. F. Barrera, and L. F. Garcia. 1997. Inhibition of virulent Mycobacterium tuberculosis by Bcgr and Bcgs macrophages correlates with nitric oxide production. J. Infect. Dis. 176:1552-1558. [DOI] [PubMed] [Google Scholar]
- 4.Aston, Ñ., W. N. Rom, A. T. Talbot, and J. Reibman. 1998. Early inhibition of mycobacterial growth by human alveolar macrophages is not due to nitric oxide. Am. J. Respir. Crit. Care Med. 157:1943-1950. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy, R. 1998. Genetic susceptibility to tuberculosis in human populations. Thorax 53:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellamy, R., C. Ruwende, T. Corrah, K. McAdam, H. C. Whittle, and A. V. S. Hill. 1998. Variations in the NRAMP1 gene and susceptibility to tuberculosis in West Africans. N. Engl. J. Med. 338:640-644. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy, R., C. Ruwende, T. Corrah, K. P. W. J. McAdams, M. Thursz, H. C. Whittle, and A. V. S. Hill. 1998. Assessment of the interleukin 1 gene cluster and other candidate gene polymorphisms in host susceptibility to tuberculosis. Tuber. Lung Dis. 79:83-89. [DOI] [PubMed] [Google Scholar]
- 8.Bloom, B. R., and C. L. Murray. 1992. Tuberculosis: commentary on a re-emergent killer. Science 257:1055-1064. [DOI] [PubMed] [Google Scholar]
- 9.Bonecini-Almeida, M. G., S. Chitale, I. Boutsikakis, J. Geng, H. Doo, S. He, and J. L. Ho. 1998. Induction of in vitro human macrophage anti-Mycobacterium tuberculosis activity: requirement for IFN-γ and primed lymphocytes. J. Immunol. 160:4490-4499. [PubMed] [Google Scholar]
- 10.Brahmajothi, V., R. M. Pitchappan, V. N. Kakkanaiah, M. Sashidhar, K. Rajaram, S. Ramu, K. Palanimurgan, C. N. Paramasivan, and R. Prabhakar. 1991. Association of pulmonary tuberculosis and HLA in South India. Tubercle 72:123-128. [DOI] [PubMed] [Google Scholar]
- 11.Byrd, T. F. 1998. Multinucleated giant cell formation induced by IFN-gamma/IL-3 is associated with restriction of virulent Mycobacterium tuberculosis cell to cell invasion in human monocyte monolayers. Cell. Immunol. 188:89-96. [DOI] [PubMed] [Google Scholar]
- 12.Chan, J., Y. Xing, R. S. Magliozzo, and B. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comstock, G. W. 1978. Tuberculosis in twins: a re-analysis of the Prophit survey. Am. Rev. Respir. Dis. 117:621-626. [DOI] [PubMed] [Google Scholar]
- 14.Cooper, A. M., A. D. Roberts, E. R. Rhoades, J. E. Callahan, D. M. Getzy, and I. M. Orme. 1995. The role of intereleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology 84:423-432. [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffen, D. G. Russel, and I. M. Orme. 1993. Disseminated tuberculosis in interferon-γ gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Jong, R., F. Altare, I. A. Haagen, D. G. Elferink, T. De Boer, P. J. C. van Breda Vriesman, P. J. Kabel, J. M. T. Draaisma, J. T. van Dissel, F. P. Kroon, J. L. Casanova, and T. H. M. Ottenhoff. 1998. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280:1435-1438. [DOI] [PubMed] [Google Scholar]
- 17.Denis, M. 1991. Killing of Mycobacterium tuberculosis within human monocytes: activation by cytokines and calcitriol. Clin. Exp. Immunol. 84:200-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobos, K. M., E. A. Spotts, F. D. Quinn, and C. H. King. 2000. Necrosis of lung epithelial cells during infection with Mycobacterium tuberculosis is preceded by cell permeation. Infect. Immun. 68:6300-6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng, C. G., A. G. Bean, H. Hooi, H. Briscoe, and W. J. Britton. 1999. Increase in gamma interferon-secreting CD8+, as well as CD4+, T cells in lungs following aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 67:3242-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenton, M. J., and M. W. Vermeulen. 1996. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect. Immun. 64:683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari, G., H. Langen, M. Naito, and J. Pieters. 1999. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97:435-447. [DOI] [PubMed] [Google Scholar]
- 22.Flesch, I., and S. H. E. Kaufmann. 1987. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J. Immunol. 138:4408-4413. [PubMed] [Google Scholar]
- 23.Flesch, I. E. A., and S. H. E. Kaufmann. 1991. Mechanisms involved in mycobacterial growth inhibition by gamma-interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect. Immun. 59:3213-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 25.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for IFN-γ in resistance to M. tuberculosis infection. J. Exp. Med. 178:2248-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 27.Goldfeld, A. E., J. C. Delgado, S. Thim, M. V. Bozon, A. M. Uglialoro, D. Turbay, C. Cohen, and E. J. Yunis. 1998. Association of HLA-DQ allele with clinical tuberculosis. JAMA 279:226-228. [DOI] [PubMed] [Google Scholar]
- 28.Greenwood, C. M., T. M. Fujiwara, L. J. Boothroyd, M. A. Miller, D. Frappier, et al. 2000. Linkage of tuberculosis to chromosome 2q35 loci, including NRAMP1, in a large aboriginal Canadian family. Am. J. Hum. Genet. 67:405-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi, T., S. P. Roa, P. R. Meylan, R. S. Kornbluth, and A. Catanzaro. 1999. Role of CD40 ligand in Mycobacterium avium infection. Infect. Immun. 67:3558-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill, A. V. S. 1996. Genetics of infectious disease resistance. Curr. Opin. Genet. Dev. 6:348-360. [DOI] [PubMed] [Google Scholar]
- 31.Jouanguy, E., F. Altare, S. Lamhamedi, R. Revy, J. F. Emile, M. Newport, M. Levin, S. Blanche, E. Seboun, A. Fischer, and J. L. Casanova. 1996. Interferon-γ-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N. Engl. J. Med. 335:1956-1961. [DOI] [PubMed] [Google Scholar]
- 32.Kramnik, I., W. F. Dietrich, P. Demant, and B. Bloom. 2000. Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 97:8560-8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lavebratt, C., A. S. Apt, B. V. Nikonenko, M. Schalling, and E. Schurr. 1999. Severity of tuberculosis in mice is linked to distal chromosome 3 and proximal chromosome 9. J. Infect. Dis. 180:150-155. [DOI] [PubMed] [Google Scholar]
- 34.Liu, J., M. Cellier, E. Schurr, and E. Skamene. 1993. Comparative genome analysis—a novel strategy to study the genetics of host-parasite interaction. J. Parasitol. 79:463-470. [PubMed] [Google Scholar]
- 35.Lyadova, I. V., E. B. Eruslanov, S. V. Khaidukov, V. V. Yeremeev, K. B. Majorov, A. V. Pichugin, B. V. Nikonenko, T. K. Kondratieva, and A. S. Apt. 2000. Comparative analysis of T lymphocytes recovered from the lungs of mice genetically susceptible, resistant and hyperresistant to Mycobacterium tuberculosis-triggered disease. J. Immunol. 165:5921-5932. [DOI] [PubMed] [Google Scholar]
- 36.Lyadova, I. V., V. V. Yeremeev, K. B. Majorov, B. V. Nikonenko, S. V. Khaidukov, T. K. Kondratieva, N. V. Kobets, and A. S. Apt. 1998. An ex vivo study of T lymphocytes recovered from the lungs of I/St mice infected with and susceptible to Mycobacterium tuberculosis. Infect. Immun. 66:4981-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacMicking, J. D., R. J. North, R. La Course, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLeod, R., E. Buschman, D. Arbuckle, and E. Skamene. 1995. Immunogenetics in the analysis of resistance to intracellular pathogens. Curr. Opin. Immunol. 7:539-552. [DOI] [PubMed] [Google Scholar]
- 39.Menozzi, F. D., R. Bischoff, E. Fort, M. J. Brennan, and C. Locht. 1998. Molecular characterization of the mycobacterial heparin-binding hemagglutinin, a mycobacterial adhesin. Proc. Natl. Acad. Sci. USA 95:12625-12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer, C. G., J. May, and K. Stark. 1998. Human leukocyte antigens in tuberculosis and leprosy. Trends Microbiol. 6:148-154. [DOI] [PubMed] [Google Scholar]
- 41.Molloy, A., and G. Kaplan. 1996. Cell-mediated immune response in tuberculosis, p. 305-326. In W. N. Rom and S. M. Garay (ed.), Tuberculosis. Little, Brown, Boston, Mass.
- 42.Newport, M., and M. Levin. 1999. Genetic susceptibility to tuberculosis. J. Infect. 39:117-121. [DOI] [PubMed] [Google Scholar]
- 43.Newport, M. J., C. M. Huxley, S. Huston, C. M. Hawrylowicz, B. A. Oostra, R. Williamson, and M. Levin. 1996. A mutation in the interferon-γ-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941-1949. [DOI] [PubMed] [Google Scholar]
- 44.Nikonenko, B. V., M. M. Averbakh, C. Lavebratt, E. Schurr, and A. S. Apt. 2000. Comparative analysis of mycobacterial infections in susceptible I/St and resistant A/Sn inbred mice. Tuber. Lung Dis. 80:15-25. [DOI] [PubMed] [Google Scholar]
- 45.Pospelov, L. E., A. G. Matrakshin, L. N. Chernousova, A. F. Malenko, V. V. Yeremeev, and A. G. Khomenko. 1996. Association of various genetic markers with tuberculosis and other lung diseases in Tuvinian children. Tuber. Lung Dis. 77:77-80. [DOI] [PubMed] [Google Scholar]
- 46.Ratazzi, C., R. D. Arbeit, C. Carini, and H. G. Remold. 1997. Programmed cell death of Mycobacterium avium serovar 4-infected human macrophages prevents the mycobacteria from spreading and induces mycobacterial growth inhibition by freshly added, uninfected macrophages. J. Immunol. 158:4320-4327. [PubMed] [Google Scholar]
- 47.Rook, G., and R. Hernandez-Pando. 1996. Pathogenesis of tuberculosis. Annu. Rev. Microbiol. 50:259-282. [DOI] [PubMed] [Google Scholar]
- 48.Rose, R. M., J. M. Fuglestad, and L. Remington. 1991. Growth inhibition of Mycobacterium avium complex in human alveolar macrophages by the combination of recombinant macrophage colony-stimulating factor and interferon-gamma. Am. J. Respir. Cell. Mol. Biol. 4:248-254. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez, F., T. V. Radaeva, B. V. Nikonenko, A.-S. Persson, S. Sengul, M. Schalling, E. Schurr, A. S. Apt, and C. Lavebratt. 2003. Multigenic control of disease severity after virulent Mycobacterium tuberculosis infection in mice. Infect. Immun. 71:126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schorey, J. S., M. C. Carroll, and E. J. Brown. 1997. A macrophage invasion mechanism of pathogenic mycobacteria. Science 277:1091-1095. [DOI] [PubMed] [Google Scholar]
- 51.Schuler, N. W., and W. N. Rom. 1998. The host immune response to tuberculosis. Am. J. Respir. Crit. Care Med. 157:679-691. [DOI] [PubMed] [Google Scholar]
- 52.Serbina, N. V., and J. L. Flynn. 1999. Early emergence of CD8+ T cells primed for production of type 1 cytokines in the lungs of Mycobacterium tuberculosis-infected mice. Infect. Immun. 67:3980-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw, M. A., A. Collins, C. S. Peacock, E. N. Miller, G. F. Black, D. Sibthorpe, Z. Lins-Lainson, J. J. Shaw, F. Ramos, F. Silveira, and J. M. Blackwell. 1997. Evidence that genetic susceptibility to Mycobacterium tuberculosis in a Brazilian population is under oligo-genetic control: linkage study of the candidate genes NRAMP1 and TNF-α. Tuber. Lung Dis. 78:35-45. [DOI] [PubMed] [Google Scholar]
- 54.Silver, R. F., Q. Li, W. H. Boom, and J. J. Ellner. 1998. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J. Immunol. 160:2408-2417. [PubMed] [Google Scholar]
- 55.Skamene, E., E. Schurr, and P. Gros. 1998. Infection genomics: Nramp 1 as a major determinant of natural resistance to intracellular infections. Annu. Rev. Med. 49:275-287. [DOI] [PubMed] [Google Scholar]
- 56.Stach, J. L., P. Gros, E. Skamene, and A. Forget. 1984. Phenotypic expression of genetically controlled natural resistance to Mycobacterium bovis (BCG). J. Immunol. 132:888-892. [PubMed] [Google Scholar]
- 57.Stead, W. W., J. W. Senner, W. T. Reddick, and J. P. Lofgren. 1990. Racial differences in susceptibility to infection by Mycobacterium tuberculosis. N. Engl. J. Med. 322:422-427. [DOI] [PubMed] [Google Scholar]
- 58.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14462. [DOI] [PMC free article] [PubMed] [Google Scholar]