Abstract
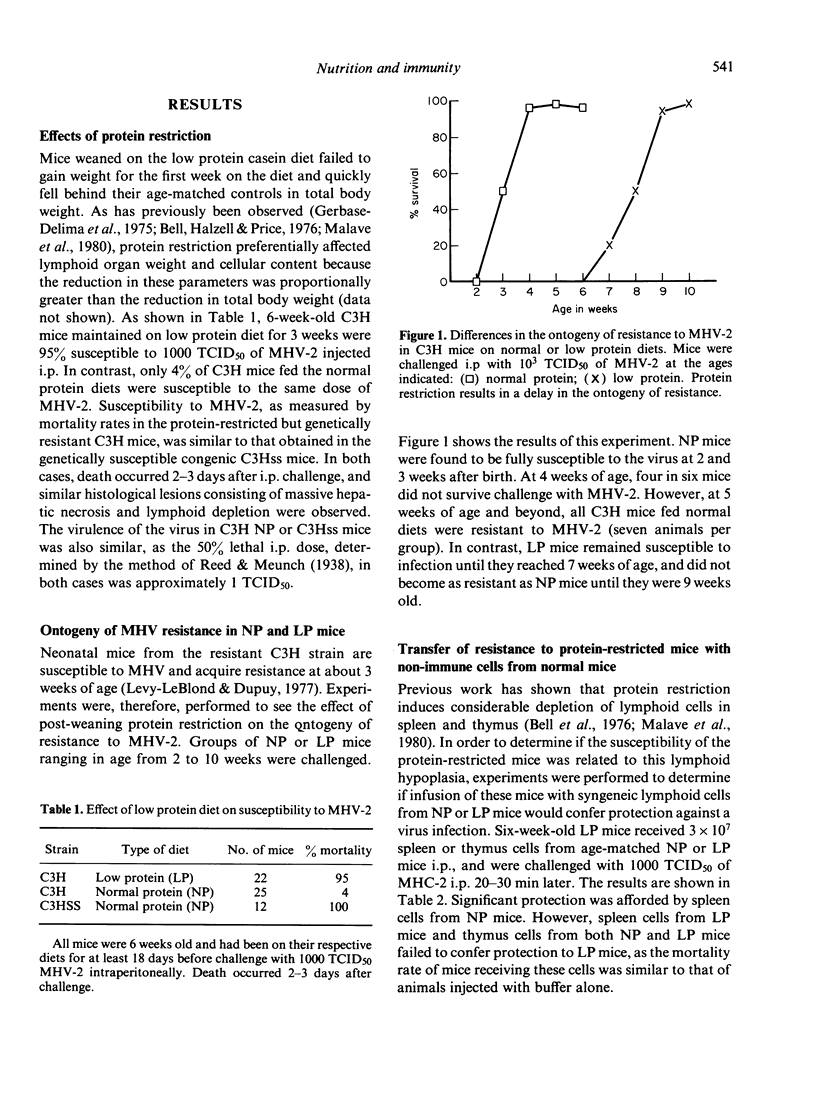
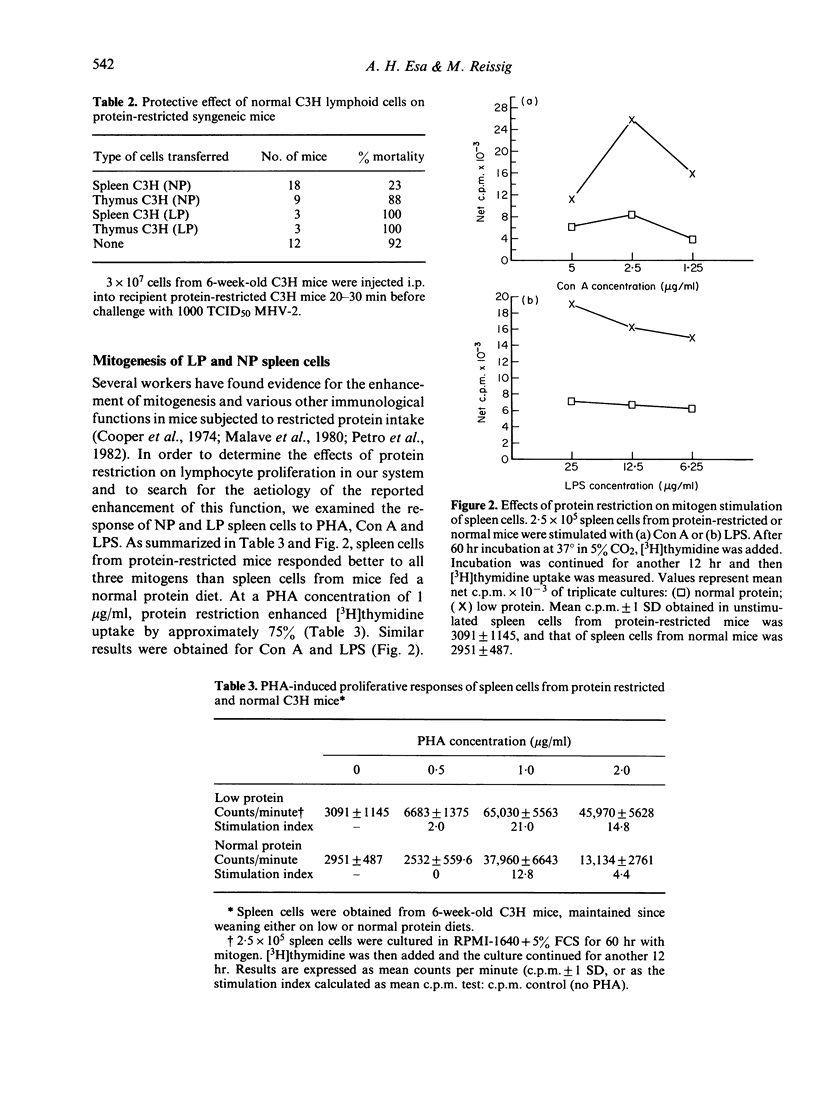
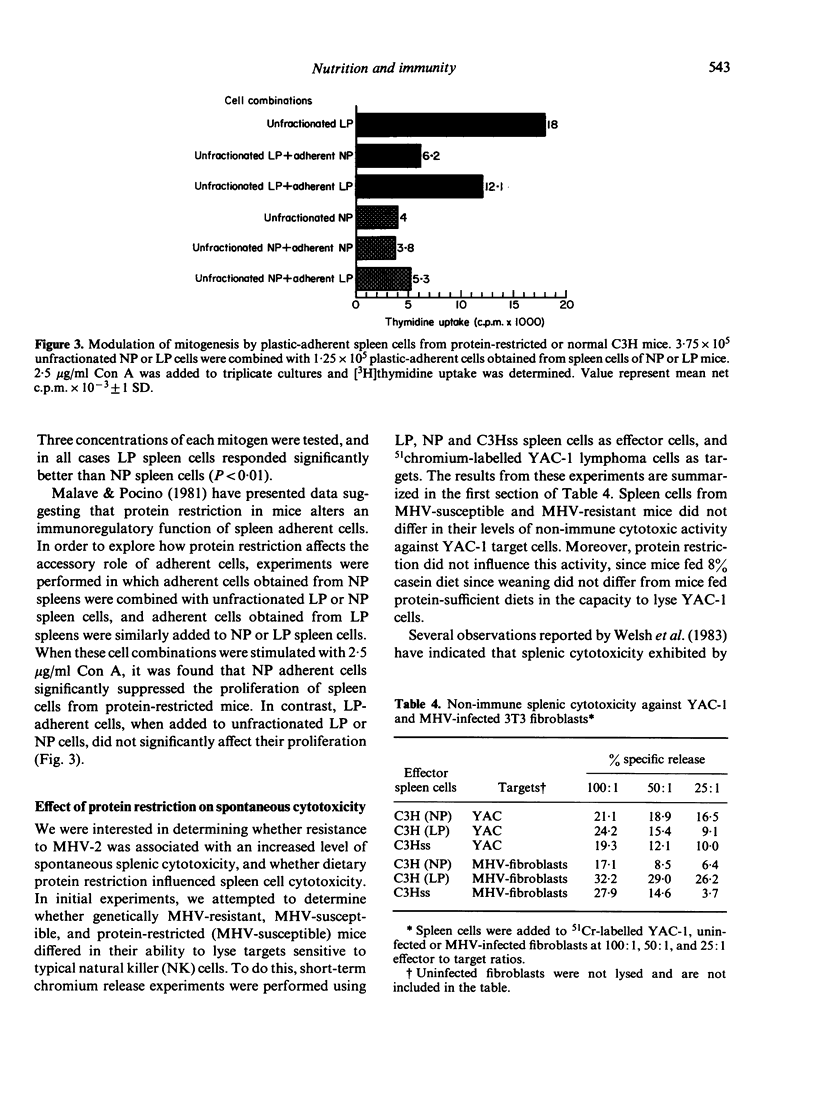
Resistance to mouse hepatitis virus (MHV) in C3H mice is a genetic trait which appears 3-4 weeks after birth. However, when these animals were weaned on a low protein diet (8% casein), they remained susceptible to MHV-2 infection until they reached 8-9 weeks of age. During this period, the protein-restricted C3H mice were as susceptible to MHV-2 as the genetically susceptible congenic C3Hss strain. The delay in the emergence of resistance in the protein-restricted mice could be corrected by injecting these animals with spleen cells from 6-week-old C3H mice. Thymocytes from normal C3H mice, and splenocytes and thymocytes from protein-restricted C3H mice, were not protective. However, spleen cells from the protein-restricted mice were more responsive to phytohaemagglutinin, lipopolysaccharide and concanavalin A than spleen cells from normal C3H. The enhanced lymphoproliferative response in spleen cells from protein-restricted mice was abrogated by the addition of plastic-adherent cells obtained from normal C3H spleens. Spleen cells from protein-restricted and from genetically susceptible C3Hss mice also possessed more spontaneous cytotoxicity against MHV-infected 3T3 fibroblasts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. G., Hazell L. A., Price P. Influence of dietary protein restriction on immune competence. II. Effect on lymphoid tissue. Clin Exp Immunol. 1976 Nov;26(2):314–326. [PMC free article] [PubMed] [Google Scholar]
- Bukowski J. F., Woda B. A., Habu S., Okumura K., Welsh R. M. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983 Sep;131(3):1531–1538. [PubMed] [Google Scholar]
- Chandra R. K. Lymphocyte subpopulations in human malnutrition: cytotoxic and suppressor cells. Pediatrics. 1977 Mar;59(3):423–427. [PubMed] [Google Scholar]
- Chandra R. K. Serum thymic hormone activity in protein-energy malnutrition. Clin Exp Immunol. 1979 Nov;38(2):228–230. [PMC free article] [PubMed] [Google Scholar]
- Chandra R. K. T and B lymphocyte subpopulations and leukocyte terminal deoxynucleotidyl-transferase in energy-protein undernutrition. Acta Paediatr Scand. 1979 Nov;68(6):841–845. doi: 10.1111/j.1651-2227.1979.tb08221.x. [DOI] [PubMed] [Google Scholar]
- Cooper W. C., Good R. A., Mariani T. Effects of protein insufficiency on immune responsiveness. Am J Clin Nutr. 1974 Jun;27(6):647–664. doi: 10.1093/ajcn/27.6.647. [DOI] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. Effect of dietary proteins and amino acids on the susceptibility of mice to bacterial infections. J Exp Med. 1958 Jul 1;108(1):69–81. doi: 10.1084/jem.108.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbase-DeLima M., Liu R. K., Cheney K. E., Mickey R., Walford R. L. Immune function and survival in a long-lived mouse strain subjected to undernutrition. Gerontologia. 1975;21(4):184–202. doi: 10.1159/000212044. [DOI] [PubMed] [Google Scholar]
- Good R. A., Fernandes G. Nutrition, immunity, and cancer-a review. Part I: Influence of protein or protein-calorie malnutrition and zinc deficiency on immunity. Clin Bull. 1979;9(1):3–12. [PubMed] [Google Scholar]
- Gross R. L., Newberne P. M. Role of nutrition in immunologic function. Physiol Rev. 1980 Jan;60(1):188–302. doi: 10.1152/physrev.1980.60.1.188. [DOI] [PubMed] [Google Scholar]
- Heresi G., Chandra R. K. Effects of severe calorie restriction on thymic factor activity and lymphocyte stimulation response in rats. J Nutr. 1980 Sep;110(9):1888–1893. doi: 10.1093/jn/110.9.1888. [DOI] [PubMed] [Google Scholar]
- Koros A. M., Axelrod A. E., Hamill E. C., South D. J. Immunoregulatory consequences of vitamin deficiencies on background plaque-forming cells in rats. Proc Soc Exp Biol Med. 1976 Jul;152(3):322–326. doi: 10.3181/00379727-152-39388. [DOI] [PubMed] [Google Scholar]
- Levy-Leblond E., Dupuy J. M. Neonatal susceptibility to MHV3 infection in mice. I. Transfer of resistance. J Immunol. 1977 Apr;118(4):1219–1222. [PubMed] [Google Scholar]
- Malavé I., Layrisse M. Immune response in malnutrition. Differential effect of dietary protein restriction on the IgM and IgG response to alloantigens. Cell Immunol. 1976 Feb;21(2):337–343. doi: 10.1016/0008-8749(76)90061-7. [DOI] [PubMed] [Google Scholar]
- Malavé I., Németh A., Pocino M. Changes in lymphocyte populations in protein--calorie-deficient mice. Cell Immunol. 1980 Feb;49(2):235–249. doi: 10.1016/0008-8749(80)90026-x. [DOI] [PubMed] [Google Scholar]
- Petro T. M., Chien G., Watson R. R. Alteration of cell-mediated immunity to Listeria monocytogenes in protein-malnourished mice treated with thymosin fraction V. Infect Immun. 1982 Aug;37(2):601–608. doi: 10.1128/iai.37.2.601-608.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUEBNER B., BRAMHALL J. L. The effect of changes in dietary protein on experimental viral hepatitis in mice. Gastroenterology. 1960 Sep;39:335–339. [PubMed] [Google Scholar]
- Schindler L., Engler H., Kirchner H. Activation of natural killer cells and induction of interferon after injection of mouse hepatitis virus type 3 in mice. Infect Immun. 1982 Mar;35(3):869–873. doi: 10.1128/iai.35.3.869-873.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Esa A., Stiff M. Transfer of Salmonella resistance and delayed hypersensitivity with murine-derived transfer factor. Infect Immun. 1982 Apr;36(1):271–276. doi: 10.1128/iai.36.1.271-276.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser W., Vellisto I., Bang F. B. Congenic strains of mice susceptible and resistant to mouse hepatitis virus. Proc Soc Exp Biol Med. 1976 Sep;152(4):499–502. doi: 10.3181/00379727-152-39426. [DOI] [PubMed] [Google Scholar]



