Abstract
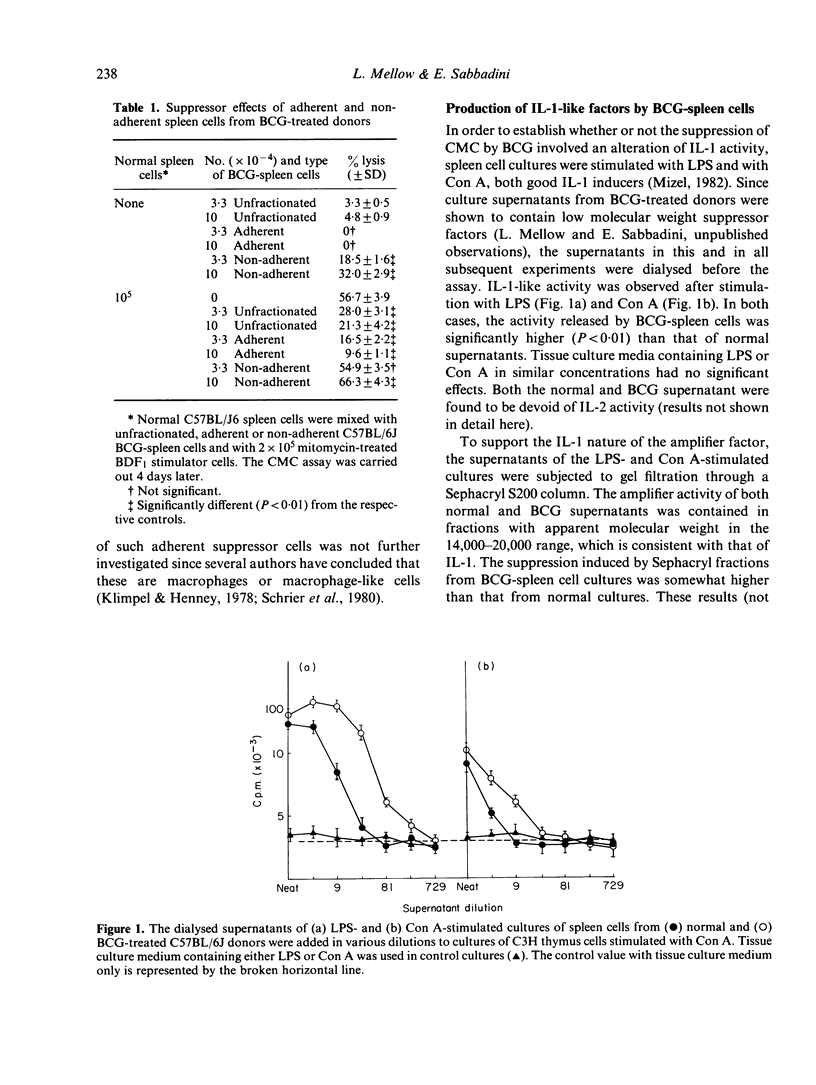
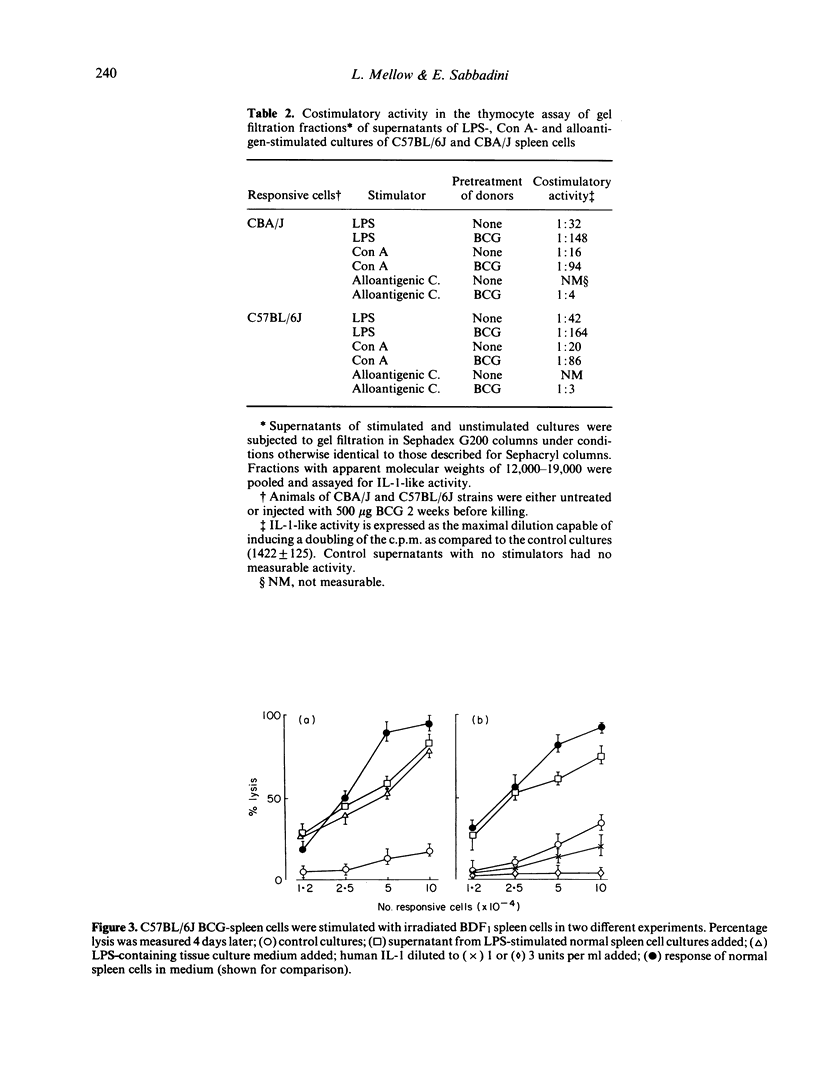
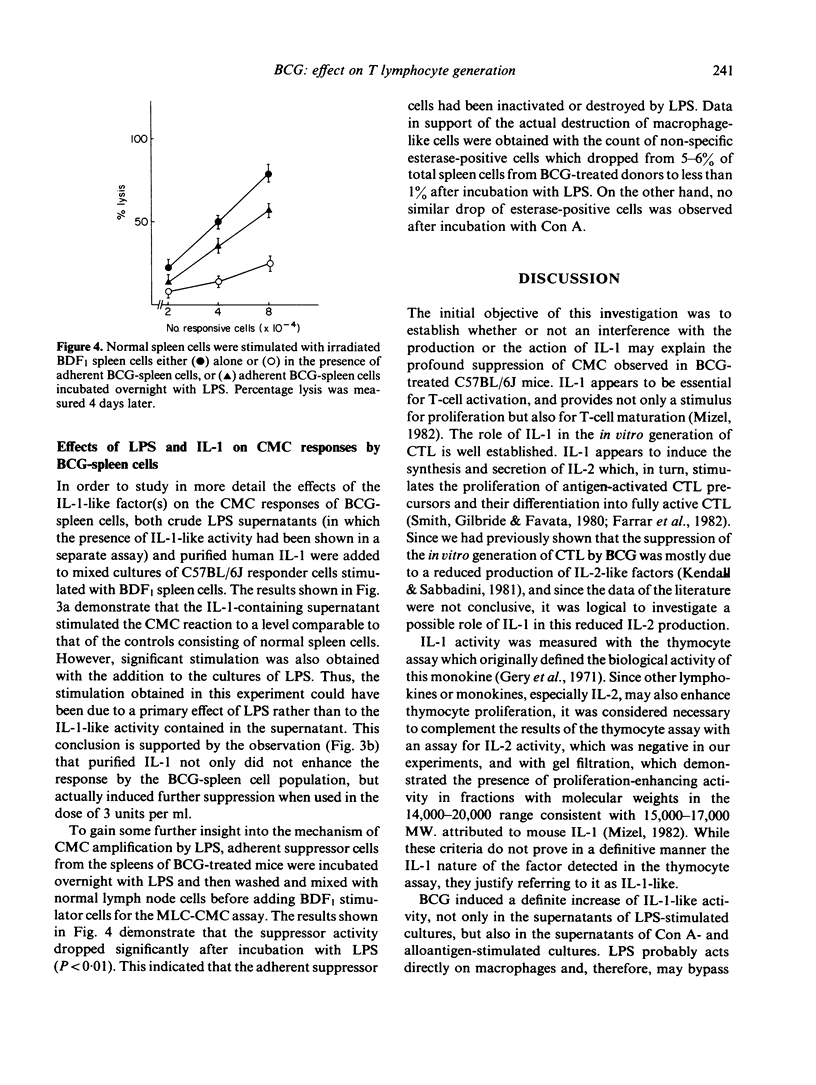
The injection of BCG vaccine in C57BL/6J mice results in the suppression of the generation of cytotoxic T lymphocytes (CTL) in mixed lymphocyte cultures (MLC) and of mitogenic reactions to concanavalin A (Con A). Suppression is mediated by macrophage-like suppressor cells. Since previous work had indicated that suppression involved the inhibition of the production of interleukin-2 (IL-2), the effects of BCG on interleukin-1 (IL-1), a monokine required for IL-2 production, were investigated. It was found that the release of IL-1-like activity in spleen cell cultures stimulated with LPS or Con A was increased by previous BCG treatment of the cell donors. In MLC, the release of IL-1-like activity was also increased by BCG. However, the detection of IL-1-like activity in MLC supernatants was prevented by the presence of a suppressor factor. In this case, the IL-1-like activity could be separated with gel filtration from the suppressor factor which had higher molecular weight. The production of IL-1-like activity by CBA/J spleen cells, which are not suppressed by BCG, was not significantly different from that of C57BL/6J cells, which are markedly suppressed. Moreover, the addition of IL-1 to the BCG-suppressed cultures not only did not restore normal reactivity, but actually further suppressed CTL formation. It was concluded that BCG-induced suppression cannot be attributed to decreased IL-1 activity. The suppressor factor discovered during these investigations may have a role in this type of suppression.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bullock W. E., Carlson E. M., Gershon R. K. The evolution of immunosuppressive cell populations in experimental mycobacterial infection. J Immunol. 1978 May;120(5):1709–1716. [PubMed] [Google Scholar]
- Castés M., Lynch N. R., Lespinats G., Orbach-Arbouys S. Possible role of macrophage-like suppressor cells in the anti-tumour activity of BCG. Br J Cancer. 1981 Dec;44(6):828–837. doi: 10.1038/bjc.1981.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar J. J., Benjamin W. R., Hilfiker M. L., Howard M., Farrar W. L., Fuller-Farrar J. The biochemistry, biology, and role of interleukin 2 in the induction of cytotoxic T cell and antibody-forming B cell responses. Immunol Rev. 1982;63:129–166. doi: 10.1111/j.1600-065x.1982.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Koopman W. J., Fuller-Bonar J. Identification and partial purification of two synergistically acting helper mediators in human mixed leukocyte culture supernatants. J Immunol. 1977 Jul;119(1):47–54. [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of cultured mouse thymocyte responses by factors released by peripheral leucocytes. J Immunol. 1971 Dec;107(6):1778–1780. [PubMed] [Google Scholar]
- Green S., Dobrjansky A., Carswell E. A., Kassel R. L., Old L. J., Fiore N., Schwartz M. K. Partial purification of a serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1976 Feb;73(2):381–385. doi: 10.1073/pnas.73.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall L., Sabbadini E. Effect of Bacillus Calmette-Guérin on the in vitro generation of cytotoxic T lymphocytes. I. Effect of BCG on the frequency of cytotoxic T lymphocyte precursors and on the production of helper factors. J Immunol. 1981 Jul;127(1):234–238. [PubMed] [Google Scholar]
- Klimpel G. R., Henney C. S. BCG-induced suppressor cells. I. Demonstration of a macrophage-like suppressor cell that inhibits cytotoxic T cell generation in vitro. J Immunol. 1978 Feb;120(2):563–569. [PubMed] [Google Scholar]
- Mitchell M. S., Kirkpatrick D., Mokyr M. B., Gery I. On the mode of action of BCG. Nat New Biol. 1973 Jun 13;243(128):216–218. doi: 10.1038/newbio243216a0. [DOI] [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Nelson D. S. Production by stimulated macrophages of factors depressing lymphocyte transformation. Nature. 1973 Nov 30;246(5431):306–307. doi: 10.1038/246306a0. [DOI] [PubMed] [Google Scholar]
- Ptak W., Zembala M., Gershon R. K. Intermediary role of macrophages in the passage of suppressor signals between T-cell subsets. J Exp Med. 1978 Aug 1;148(2):424–434. doi: 10.1084/jem.148.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier D. J., Allen E. M., Moore V. L. BCG-induced macrophage suppression in mice: suppression of specific and nonspecific antibody-medicated and cellular immunologic responses. Cell Immunol. 1980 Dec;56(2):347–356. doi: 10.1016/0008-8749(80)90110-0. [DOI] [PubMed] [Google Scholar]
- Schrier D. J., Sternick J. L., Allen E. M., Moore V. L. Immunogenetics of BCG-induced anergy in mice: control by genes linked to the Igh complex. J Immunol. 1982 Mar;128(3):1466–1469. [PubMed] [Google Scholar]
- Scott M. T. Biological effects of the adjuvant Corynebacterium parvum. II. Evidence for macrophage-T-cell interaction. Cell Immunol. 1972 Nov;5(3):469–479. doi: 10.1016/0008-8749(72)90073-1. [DOI] [PubMed] [Google Scholar]
- Tadakuma T., Pierce C. W. Mode of action of a soluble immune response suppressor (SIRS) produced by concanavalin a-activated spleen cells. J Immunol. 1978 Feb;120(2):481–486. [PubMed] [Google Scholar]
- Turcotte R. Evidence for two distinct populations of suppressor cells in the spleens of Mycobacterium bovis BCG-Sensitized mice. Infect Immun. 1981 Nov;34(2):315–322. doi: 10.1128/iai.34.2.315-322.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R., Lemieux S. Mechanisms of action of Mycobacterium bovis BCG-induced suppressor cells in mitogen-induced blastogenesis. Infect Immun. 1982 Apr;36(1):263–270. doi: 10.1128/iai.36.1.263-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Hardt C., Heeg K., Pfizenmaier K., Solbach W., Bartlett R., Stockinger H., Röllinghoff M. T-T cell interactions during cytotoxic T lymphocyte (CTL) responses: T cell derived helper factor (Interleukin 2) as a probe to analyze CTL responsiveness and thymic maturation of CTL progenitors. Immunol Rev. 1980;51:215–255. doi: 10.1111/j.1600-065x.1980.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Waldman S. R., Gottlieb A. A. Macrophage regulation of DNA synthesis in lymphoid cells: effects of a soluble factor from macrophages. Cell Immunol. 1973 Oct;9(1):142–156. doi: 10.1016/0008-8749(73)90175-5. [DOI] [PubMed] [Google Scholar]



