Abstract
A major allergen of the lymphatic filarial nematode Brugia malayi, a homologue of γ-glutamyl transpeptidase (γ-GT), is involved in the pathology of tropical pulmonary eosinophilia (TPE) through its potent allergenicity and the induction of antibodies against the host pulmonary epithelium. To investigate the immunoglobulin G (IgG) subclass and IgE responses to recombinant B. malayi γ-GT, we analyzed the results obtained from 51 patients with differing clinical manifestations of bancroftian filariasis. γ-GT-specific IgG1, rather than IgG4, was the predominant IgG subclass, particularly in patients with TPE (geomean, 6,321 ng/ml; range, 78 to 354,867 ng/ml) and was 75 times higher than in patients with elephantiasis (CP) (P < 0.003) and 185 times higher than in endemic normal individuals (ENL) (P < 0.010). IgG2 responses were low and IgG3 was almost absent, with no significant differences among the groups. γ-GT-specific IgG4 responses were significantly elevated in those with subclinical microfilaremia (MF) compared to the CP and ENL groups and correlated with the presence of circulating filarial antigen (CAg). More significantly, γ-GT-specific IgE antibody levels were strikingly elevated in patients with TPE (geomean, 681 ng/ml; range, 61 to 23,841 ng/ml) and in the ENL group (geomean, 106 ng/ml; range, 13 to 1,405 ng/ml) whereas the γ-GT-specific IgE level was 44 and 61 times lower in those with MF and CP, respectively (P < 0.001). Elevated γ-GT-specific IgE/IgG4 ratios were demonstrated in patients with TPE (ratio, 45) and ENL (ratio, 107). Because expression of γ-GT in Brugia infective third-stage larvae (L3) was demonstrated by immunoblot analysis, the elevated γ-GT-specific IgE antibodies appear to be associated not only with pulmonary pathology but also with possible resistance to infection in lymphatic filariasis.
A common feature between atopic diseases and human infection with the lymphatic filarial nematodes Wuchereria bancrofti, Brugia malayi, or Brugia timori is the potent elevation of total and specific immunoglobulin E (IgE) and IgG4 antibody responses (18, 20, 26, 40). While the involvement of allergen-specific IgE in the pathophysiology of bronchial asthma and atopic diseases is clearly documented (2), the role of parasite-specific IgE in helminth diseases is under debate. Evidence from animal models (5) and from longitudinal epidemiological studies of patients infected with Schistosoma spp. has linked parasite-specific IgE to resistance to reinfection and has associated parasite-specific IgG4 antibodies with increased susceptibility (9, 10, 15, 46). However, the potent effector function of IgE antibodies occurring through interaction with specific surface receptors (Fcɛ receptors I and II) present on a wide variety of inflammatory cells can result in inflammatory reactions associated with pathologic changes (3). Although high levels of total and parasite-specific IgE are common in patients with helmintic infections, manifestations of clinical allergy are less common. The presence of elevated concentrations of IgG4 antibodies is thought to modulate the IgE-mediated allergic responses due to a “blocking activity” resulting from the parallel antigen recognition by IgE antibodies and thus cause a possible interference with mast cell-bound IgE, which might functionally block IgE-mediated hypersensitivity reactions in vivo (20, 22). These blocking antibodies are part of the anti-inflammatory network, which includes the production of down-regulatory molecules including interleukin-10 IL-10, and transforming growth factor β (38, 55).
Numerous cross-sectional and longitudinal studies carried out with patients with lymphatic filariasis support the notion that the magnitude and kinetics of, and ultimately the ratio between, the IgE and IgG4 isotypes are important to the clinical outcome of infection. Elevated ratios of filaria-specific IgE to IgG4 have been found in patients with chronic lymphatic disease when total soluble filarial protein extracts were used; in contrast, microfilaremic patients (MF) without any manifestation of allergic disease have high levels of IgG4 and hence a low IgE/IgG4 antibody ratio (19, 21, 22, 27, 53).
In animal models, infection with nematodes increases the levels of total and parasite-specific IgG1 and IgE antibodies, both of which are implicated in hypersensitivity reactions. However, this property is not dependent on the exact infection since soluble extracts from Ascaris suum (28, 49), Nippostrongylus brasiliensis (11), Toxocara canis (8), and Brugia (44) have been reported to induce Th2 CD4+ responses. Thus, these observations imply that nematode-derived molecules specifically induce cytokines or cytokine-like products that preferentially induce Th2 responses. Despite the previously noted bias of chronic helminth infections toward a Th2 response, only limited knowledge of the molecular nature of nematode allergens is available. It is likely that structural features in the nematode proteins preferentially induce IgE and IgG4; thus, elucidation of the structural basis of allergens will clarify their interplay with the immune system and genetic control by the host. Several of the few well-characterized filarial allergens are members of the nematode polyprotein allergen gene family (NPA) (25). Patients with brugian filariasis preferentially develop IgE, IgG4, and IgG2 antibodies to gp15/400, an NPA member present in Brugia and Wuchereria filarial worms (54), and specific IgE to recombinant gp15/400 (with a mean level of 3.2 ng/ml) has been detected in patients with chronic disease (43).
In an effort to define the molecular basis for the pronounced allergenic properties of helminth-derived molecules, we have characterized allergens involved in the pathology of tropical pulmonary eosinophilia (TPE) and capable of inducing systemic and, most importantly, local IgE responses in the lungs of patients with TPE (29). Using affinity-purified IgE antibodies from patients with acute TPE against this major filarial allergen, we cloned a cDNA from B. malayi encoding a homologue of human γ-glutamyl transpeptidase (γ-GT) (30), a multifunctional enzyme that plays a key role in the metabolism of glutathione. We have also shown that recombinant B. malayi γ-GT strongly binds IgE from patients with TPE and have detected the presence of a similar allergen in protein extracts from W. bancrofti adult filarial worms (31). In addition, we have demonstrated that in BALB/c mice this recombinant filarial antigen induced pronounced elevation of IgG1, IgA, and IgE levels, even in the absence of adjuvants. Furthermore, we have shown that intranasal challenge with B. malayi γ-GT in previously sensitized mice induced pulmonary inflammation and humoral autoimmunity toward the endogenous γ-GT on the pulmonary epithelium (13).
In this study, we have quantified the specific IgE and IgG subclass antibody responses to recombinant B. malayi γ-GT allergen in 51 patients with different manifestations of lymphatic filariasis and have correlated them with the different clinical manifestations of the disease. Because of the importance of the balance of IgE and IgG4 isotypes in the development of helminth immunity, control of allergic reactions, and the development of disease, we have analyzed the IgE/IgG ratios and correlated them with the clinical status. Here we report a correlation of strikingly elevated levels of specific IgE to B. malayi γ-GT and markedly increased specific IgE/IgG4 ratios in patients with pulmonary disease. We also report the presence of this potent allergen in Brugia infective third-stage larvae (L3) and its immune recognition by infection-free endemic control individuals.
MATERIALS AND METHODS
Purification of His-tagged B. malayi γ-GT protein.
The C-terminally histidine-tagged B. malayi γ-GT precursor (amino acids 25 to 590) cloned in the vector pJC20 was expressed in Escherichia coli BL21(DE3). Soluble B. malayi γ-GT protein was isolated as described previously (30). Protein yields were measured by bicinchoninic acid protein determination (Pierce, Rockford, Ill.).
Determination of the total IgE level.
The total IgE antibody concentration in the standard serum pool (made from sera of patients selected from a group of 80 with lymphatic filariasis) was assayed by the Clinical Pathology Laboratory, National Institutes of Health, Bethesda, Md., and determined to be 132,744 IgE ng/ml.
Patient population.
Fifty-one patients with W. bancrofti infection were studied. All were Indian adults living in the region of Madras (South India), where lymphatic filariasis is endemic. Patients were categorized into four clinical groups with clear distinguishing features. Endemic normals (ENL; n = 8), who had resided for their whole lives in the same area of endemic infection, had no current historical, parasitological, or physical evidence of infection with lymphatic filariasis and had no detectable circulating filarial antigen (CAg) in their serum, determined as described previously (35); microfilaremic patients (MF; n = 17) had microfilaremia; patients with chronic pathology (CP; n = 15) had either lymphedema (elephantiasis) or hydrocele of more than 2 years' duration; patients with tropical pulmonary eosinophilia (TPE; n = 11) showed all the clinical characteristics of this filarial syndrome including nocturnal asthma, wheezing, pulmonary infiltrates, extreme blood and pulmonary eosinophilia, and very high total IgE and filaria-specific IgG antibody titers (41). The analysis included 10 North American control individuals with no previous history of any parasitic nematode infection.
Enzyme-linked immunosorbent assay ELISA for B. malayi γ-GT-specific IgG subclasses.
To measure the levels of specific Igs to recombinant B. malayi γ-GT, 96-well flat-bottom plates (Immunolon IV; Dynatech, Boston, Mass.) were coated with 1 μg of recombinant B. malayi γ-GT per ml in 50 μl of 0.1 M carbonate-bicarbonate buffer (pH 9.6) for IgG1 and IgG3 and with 2 μg/ml for IgG2 and IgG4 for 1 h at 37°C and then overnight at 4°C. Subsequently, the plates were washed and blocked with 5% bovine serum albumin-0.05% Tween 20 in phosphate-buffered saline for 2 h and washed again. The absolute levels of specific IgG subclass were determined using a reference pool with known quantities of B. malayi γ-GT-specific IgG, previously determined by heterologous interpolation (40). The standard serum pool, with known concentrations of each B. malayi γ-GT-specific IgG subclass, was used in each experiment over a range of dilutions (1:100 to 1:12,800). The plates were incubated overnight at 4°C with 50 μl of serially diluted sample sera (1:100 and 1:500 for IgG1, 1:10 and 1:500 for IgG2, 1:10 and 1:200 for IgG3, and 1:100 and 1:500 for IgG4) in duplicate. After being washed, the plates were incubated for 2 h with mouse anti-human IgG1 monoclonal antibody at 1:1,000 (6069), anti-IgG2 at 1:2,000 (6002), anti-IgG3 at 1:1,000 (6047), or anti-IgG4 at 1:1,000 (6023) as described previously (40). After a further wash, the plates were incubated for 1 h at 37°C with 50 μl of alkaline phosphatase-conjugated goat anti-mouse IgG (Fcγ at 1:500; Jackson ImmunoResearch Laboratories, West Grove, Pa.). Finally, the plates were washed and developed with 1 mg of p-nitrophenyl phosphate disodium/ml (Sigma Chemical, St. Louis, Mo.). The reaction was stopped with 3 N NaOH, and the absorbance at 405 nm was determined in a microplate reader.
ELISA for B. malayi γ-GT-specific IgE.
Blocking IgG antibodies were removed by preadsorption to protein G-Sepharose overnight at 4°C as previously described (29). The concentration of B. malayi γ-GT-specific IgE antibodies in the filariasis serum pool used as a reference was determined by heterologous interpolation (40) as follows. Immunoplates (96 wells) (Immunolon IV) were coated with 10 μg of rabbit anti-human IgE/ml and with 1 μg of recombinant B. malayi γ-GT/ml in 50 μl of 0.1 M carbonate-bicarbonate buffer (pH 9.6) for 1 h at 37°C and subjected to overnight incubation at 4°C. The plates were washed six times and saturated with phosphate-buffered saline-5% bovine serum albumin as described above, for 1 h at 37°C. After being washed, the plates were incubated overnight with a serially diluted standard IgE (with known IgE concentration and with the filariasis serum pool). The plates were washed again, biotinylated mouse anti-human IgE (1:2,500) (clone 7.12; a kind gift from Andrew Saxon, University of California, Los Angeles) was added, and the plates were incubated for 2 h at 37°C, washed, and incubated with extrAvidin alkaline phosphatase (1:1,000) for 1 h at 37°C. Bound antibody was revealed by incubation with p-nitrophenylphosphate. The levels of γ-GT-specific IgE antibodies in the patient population studied were determined on plates coated with 1 μg of recombinant B. malayi per ml as described above, using serially diluted samples (1:5, 1:15, and 1:45). The plates were saturated, incubated with serially diluted IgG preadsorbed sera (1:20, 1:50, and 1:100) overnight at 4°C, and processed as described above. For each plate, a standard curve of a high-titer standard serum pool with known concentrations of filarial γ-GT-specific IgE was included. The amount of γ-GT-specific IgE antibodies in each serum was calculated (in nanograms per milliliter) by comparison with the filariasis serum pool.
Western blot analysis of infective larval stage L3.
Soluble proteins (30 μg/lane) from L3 B. malayi were prepared as described previously (12), resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12.5% polyacrylamide), and transferred onto nitrocellulose membranes for subsequent Western blot analysis (30). Specific binding of mouse anti-recombinant B. malayi γ-GT antibodies (1:3,000) was performed overnight at 4°C. After being washed, the membranes were incubated for 1 h with horseradish peroxidase-conjugated goat anti-rabbit antibody diluted 1:5,000 (Vector Laboratories, Burlingame, Calif.) at room temperature. After washing, immunolabeling was visualized with an ECL kit as specified by the manufacturer (Amersham).
Statistics.
Data are expressed as geometric means. The Mann-Whitney U test was performed for comparison of grouped data, with the significance level set at P < 0.01. Geometric means are presented for ratios of specific IgE to specific IgGs. Correlations of groups of ordered data were determined by Spearman rank correlation.
RESULTS
Analysis of the differential antibody responses to recombinant B. malayi γ-GT in patients infected with W. bancrofti.
Having demonstrated qualitative recognition of recombinant B. malayi γ-GT by IgE antibodies from either single patients or a pool of patients with TPE by immunoblot analysis (29-31), we next quantitatively analyzed the IgE and IgG isotype reactivity to recombinant B. malayi γ-GT in 51 patients from an area in South India where W. bancrofti is endemic. The general characteristics of the studied population are shown in Table 1.
TABLE 1.
Summary of the study population
| Clinical group | No. of patients
|
Age (yr)
|
No. with circulating filarial antigen (−/+) | |||
|---|---|---|---|---|---|---|
| Total | Male | Female | Mean | Range | ||
| Endemic controls | 8 | 5 | 3 | 25 | 18-30 | 8/0 |
| Microfilaremics | 17 | 14 | 3 | 32 | 16-47 | 1/16 |
| Chronic pathology | 15 | 8 | 7 | 41 | 21-61 | 5/10 |
| TPE | 11 | 11 | 0 | 29 | 16-48 | 6/5 |
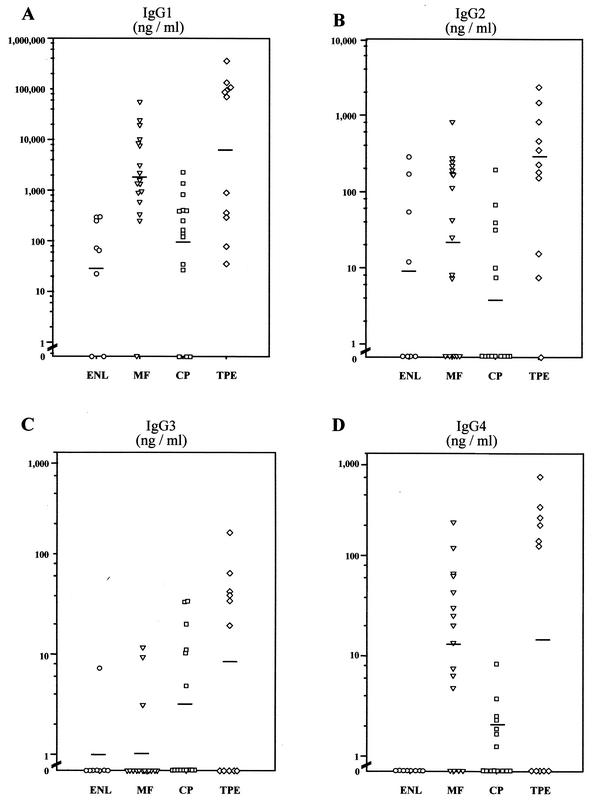
Analysis of the B. malayi γ-GT-specific IgG1 subclasses revealed a predominance of the IgG1 immune response over the other subclasses (Fig. 1). Highly elevated levels of IgG1 antibodies were demonstrated in patients with acute TPE, with a geometric mean (GM) of 6,321 ng/ml, levels significantly different from the MF group (GM, 1,744 ng/ml). The ENL group had significantly lower IgG1 means (GM, 33.9 ng/ml) than did the MF and TPE groups (both P < 0.01), whereas the CP group showed considerably higher IgG1 responses (GM, 84.5 ng/ml). All TPE patients and 94% of MF patients mounted an IgG1 response to B. malayi γ-GT, compared to 75 and 80% of ENL and CP patients, respectively. Interestingly, a dichotomous response in specific IgG1 was observed among the TPE patients, (Fig. 1A). Four of the five patients with a relatively low response were CAg negative (CAg−) whereas five of the six high responders were CAg+.
FIG. 1.
Quantitation of B. malayi γ-GT-specific IgG of each subclass for individuals in each clinical group with W. bancrofti filariasis: ENL (n = 8), MF (n = 15), CP (n = 17), and TPE (n = 11) as measured by ELISA. Horizontal bars denote the GM. Background values (GM + 2 standard deviations) obtained from 10 normal individuals with no previous exposure to any parasitic nematodes have been subtracted from all values and are as follows: IgG1 = 1,537.4, IgG2 = 74.1, IgG3 = 20.8, and IgG4 = 0. Corrected P values were used for significant differences (Mann-Whitney U test). (A) The level of specific IgG1 antibody to B. malayi γ-GT was significantly different in the TPE and MF groups from in the ENL group (P < 0.006 and P < 0.0036 respectively) and between the MF and CP groups (P < 0.008). (B) The level of specific IgG2 antibody differed significantly only between the TPE and CP groups (P < 0.0013). (C) No significant differences in the specific IgG3 levels between any groups were detected (P > 0.01). (D) The level of specific IgG4 to the recombinant allergen was significantly different between the MF and the ENL groups (P < 0.0017) and between the MF and the CP groups (P < 0.002).
When the mean levels of γ-GT-specific IgG2 antibodies were compared, a significant difference (P < 0.001) was observed only between the TPE (GM, 135.2 ng/ml) and CP (GM, 3 ng/ml) groups (Fig. 1B). The IgG3 antibody response to B. malayi γ-GT was the most restricted of the IgG subclass immune responses. The concentrations of IgG3-specific antibodies were very low in all groups. Interestingly, the subclass analysis revealed only a relatively moderate increase in the level of specific IgG4 (Fig. 1D). Most notably, all ENL individuals were IgG4 negative, lending credence to the concept that IgG4 antibodies may be a marker for active filarial infection (27, 40). Of the MF patients, 94% had detectable IgG4 antibody responses, responses comparable to those of the TPE group. Just as with IgG1, the IgG4 levels in the TPE group were dichotomous, with those being negative for IgG4 being CAg−.
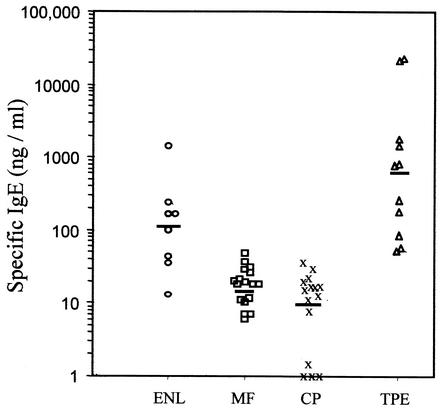
The most striking finding of this study was the demonstration of extraordinarily elevated concentrations of specific IgE against B. malayi γ-GT in all patients with TPE (GM, 681 ng/ml) (Fig. 2; Table 2). These allergen-specific immune IgE responses represent the most potent allergic response to any antigen currently described. In 7 of 11 TPE patients with extremely elevated IgE concentrations (>500 ng of IgE/ml), the mean specific IgE level was 2,528 ng/ml, which was significantly higher than in patients with CP or MF. Surprisingly, the ENL group exhibited a pronounced and significant elevation of γ-GT-specific IgE antibodies (GM, 106.3 ng/ml) compared to either the CP or MF groups (GM, 11.2 and 15.3 ng/ml, respectively; P < 0.001 for both comparisons). No relationship was found between the levels of specific IgE and the levels of circulating antigen or the age of the patients (data not shown).
FIG. 2.
Levels of B. malayi γ-GT-specific IgE in the sera of 51 patients with bancroftian lymphatic filariasis in the four different clinical groups as measured by ELISA. Results are expressed as nanograms of specific IgE to recombinant B. malayi γ-GT per milliliter. Horizontal bars indicate the geometric means. No IgE reactivity to recombinant B. malayi γ-GT was found in the 10 ENL individuals who had no previous exposure to any parasitic nematode. Significant differences in the levels of specific IgE to recombinant B. malayi γ-GT were found between the TPE and MF groups (P < 0.001) and between the TPE and CP groups (P < 0.001), as well as between the ENL and CP groups (P < 0.004).
TABLE 2.
IgE to B. malayi γ-GT
| Clinical group | IgE (ng/ml)a
|
|
|---|---|---|
| Specific | Total | |
| Endemic controls | 106.3* (13-1,405) | 146.0 (49-220) |
| Microfilaremics | 15.3 (6-60) | 1,301.4 (33-8,800) |
| Chronic pathology | 11.2 (0-48) | 1,766.3 (199-7,970) |
| TPE | 681* (55-23,841) | 20,536.4 (8,238-46,795) |
Data are the geometric means for each group, with minimal and maximal values.
IgE responses to B. malayi γ-GT were significantly higher in the TPE group than in the MF and CP groups for the Mann Whitney test (P < 0.001) and between the ENL versus CP groups (P < 0.001).
Although there was no significant correlation between the levels of IgE and IgG4 among individuals with MF or CP, there was a significant (r = 0.82, P < 0.01) correlation between γ-GT-specific IgE and IgG4 in those with TPE. There was an equally strong relationship between γ-GT-specific IgE and IgG1 in those with TPE.
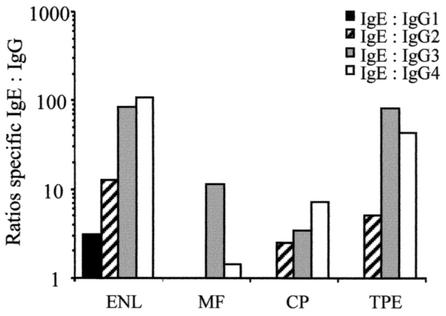
Since it has been suggested that the balance between the IgE and IgG4 isotypes plays an important role in the modulation of allergic activity and in resistance to infection (9, 10, 15, 46), we compared the concentrations of B. malayi γ-GT-specific IgE to all IgG subclasses among the four clinical groups (Fig. 3). IgE/IgG1 ratios were low in all the groups, demonstrating the dominance of the IgG1 subclass response. Analysis of the IgE/IgG2 ratios showed low ratios in the ENL and TPE groups (12.4 and 5.0, respectively). In contrast, very high IgE/IgG3 ratios were observed in ENL and TPE patients, with both groups having an elevated ratio of 84. Most important, elevated filarial γ-GT-specific IgE/IgG4 ratios of 107 and 46 were observed in the ENL and TPE groups, respectively. By contrast, far lower IgE/IgG4 ratios were seen in the other clinical groups.
FIG. 3.
Specific IgE/IgG1, IgE/IgG2, IgE/IgG3, and IgE/IgG4 ratios to recombinant B. malayi γ-GT in 51 patients with bancroftian filariasis from an area of endemic infection in South India. The bars represent the GM of the respective individual IgE/IgGs ratios.
The elevated concentrations of B. malayi γ-GT-specific IgE present in the ENL group prompted us to reexamine the presence of γ-GT in protein extracts from infective larvae (L3) by use of a hyperimmune mouse serum raised against recombinant B. malayi γ-GT and mouse preimmune serum as a control (Fig. 4). Expression of this filarial allergen in L3 was confirmed by detection of a main antigen migrating with a molecular mass of approximately 58 kDa and minor bands between 52 and 66 kDa, corresponding to the already described γ-GT glycosylated heavy chain (13, 30) (Fig. 4, lane 1). No specific reaction to any of these proteins was observed with control preimmune serum (lane 2).
FIG. 4.
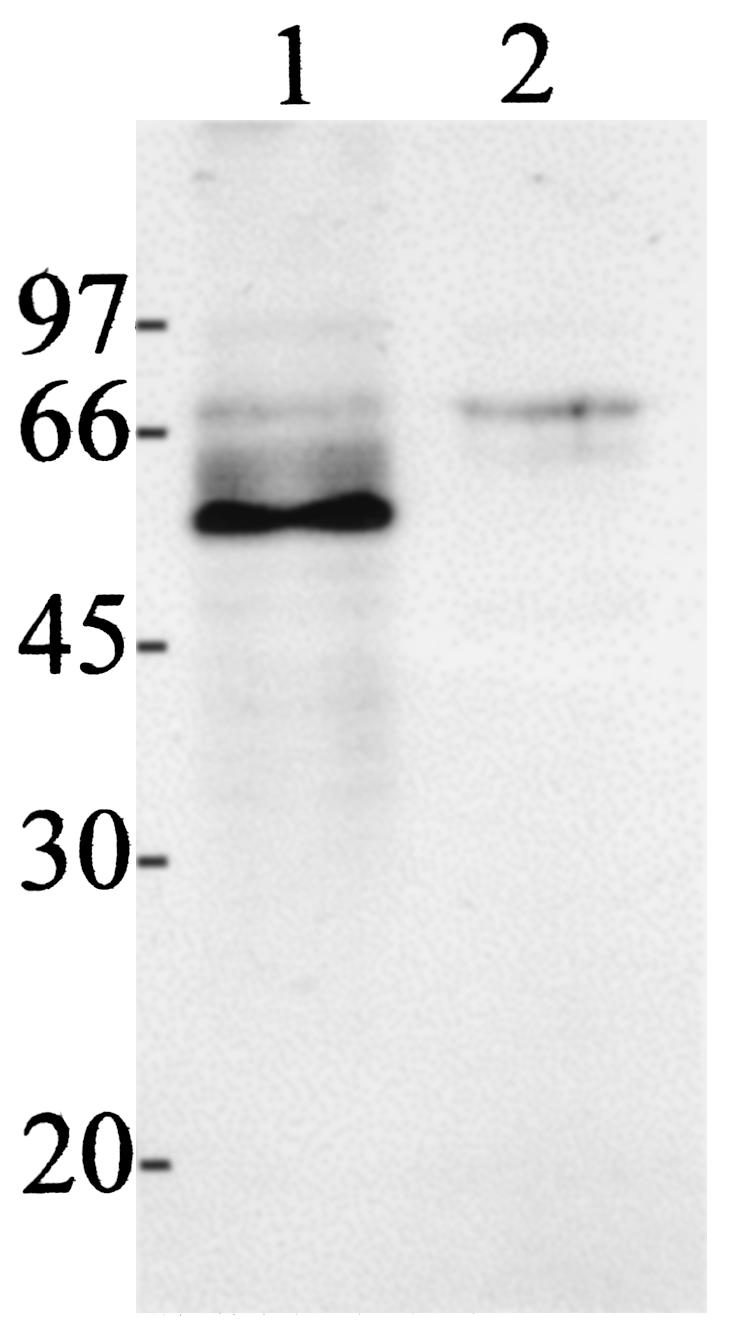
Expression of γ-GT in B. malayi L3 larvae. Soluble L3 protein at 30 μg/lane was separated by denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12.5% gels, and an immunoblot analysis was carried out as described previously (30). Lanes 1, incubation with mouse anti-B. malayi γ-GT (1:3,000); 2, incubation with mouse preimmune serum (1:3,000).
DISCUSSION
In lymphatic filariasis and other helminth infections, expansion of the IL-4-, IL-5-, and IL-13-producing CD4+ cell subset which regulates the polyclonal and antigen-specific IgE and IgG4 production, eosinophilia, and mastocytosis is well documented (38, 55). Because of the potential roles of IgE in both the disease and protective immunity to infection, we analyzed quantitatively the immune responses to the B. malayi homologue of γ-GT and determined if a particular pattern of isotype recognition was associated with a clinical outcome.
Remarkable findings from the quantitative analysis of the IgG subclasses to B. malayi γ-GT were the predominance of B. malayi γ-GT-specific IgG1 and the dichotomous IgG1 immune response observed in patients with TPE, with one group having extremely elevated levels and one having lower IgG1 levels. The pronounced allergen-specific IgG1 stands in contrast to the predominance of IgG4 immune responses to crude filarial extracts in patients with lymphatic filariasis and/or to defined recombinant filarial allergens such as Bpl-4, a subunit of gp15/400 (19, 21, 26, 54). This finding raises questions about the precise role of allergen-specific IgG1 and suggests that IgG1 is likely to be involved in the pulmonary allergic reactions of the TPE syndrome. Indeed, allergen-specific IgG1, through interaction with the Fcγ RII receptor, was recently shown to induce eosinophil degranulation in vitro in humans (23). Surprisingly, a markedly elevated IgG1 immune response to filarial γ-GT was demonstrated in the majority of individuals with MF, indicating that the previously described state of immune hyporesponsiveness among these patients, characterized by reduced levels of filaria-specific IgG and IgE antibody production (32, 36), is clearly restricted to certain filarial antigens. The IgG1 and IgG4 levels were related to the presence of an active infection, since five of six TPE patients with low specific IgG1 and all IgG4-negative TPE patients were CAg−. All ENL patients were CAg− and had no specific IgG4. A large proportion of microfilaremic individuals (75%) mounted a strong IgG4 response to B. malayi γ-GT, confirming that filaria-specific IgG4 may be a marker for active infection (27). Weak IgG4 responses were observed in patients with elephantiasis and hydrocele, although 10 of the 15 patients were positive in the circulating antigen test and thus were infected. IgG2-specific antibody levels were low; IgG2 antibodies are the predominant IgG subclass induced by carbohydrates (48). The native B. malayi γ-GT is glycosylated (30), which may explain the induction of IgG2 antibody responses in the TPE and ENL groups. Alternatively, IgG2 reactivity may result from structural mimicry between peptides and carbohydrate moieties (24, 52). IgG3 responses to filarial γ-GT were practically absent, and no significant differences among the four groups were found, consistent with reports showing an absence of filaria-specific IgG3 in patients infected with W. bancrofti from South India (40) but not with others showing elevated IgG3 levels in patients with elephantiasis (53).
The high levels of not only IgG1, controlled by Th1 cytokines, but also IgE, controlled by Th2 cytokines, induced by B. malayi γ-GT observed in patients with TPE are in agreement with the recently described expansion of human CD4+ helper cells from patients with TPE with a mixed phenotype secreting gamma interferon, IL-2, IL-4, and IL-5 (38). A mixed pattern of cytokines was induced by recombinant B. malayi γ-GT in a BALB/c mouse model of pulmonary inflammation, which resulted in high levels of specific IgG1, IgA, and IgE (Th2 dependent) and of specific IgG2 (Th1 dependent) (13). The strikingly elevated B. malayi γ-GT-specific IgE antibody levels in patients with TPE are among the highest specific IgE levels currently quantified against a given allergen and are several logarithmic units higher than the specific IgE levels determined for allergens in patients with atopic conditions. A role for the pulmonary filaria-specific IgE in the pathogenesis of TPE is strongly suggested, since the decline of parasite-specific IgE levels in the bronchoalveolar lavage fluid after chemotherapy parallels clinical improvement (37). The clear-cut differences in B. malayi γ-GT-specific IgE concentrations between the TPE and both the MF and CP groups define IgE concentration as a specific marker of pulmonary disease.
Patients with chronic lymphatic obstruction and hydrocele showed little elevation in the level of B. malayi γ-GT-specific IgE. This result is in contrast to the results of studies showing that patients with CP had either a significantly higher percentage of filaria-specific IgE antibodies against total parasite extract and elevated IgE/IgG4 ratios (40) or uniformly elevated levels of specific IgE against the filarial allergen gp15/400 compared to other clinical groups (43). Our results suggest that B. malayi γ-GT-specific IgE makes a small or no contribution to the late-stage pathologic changes of lymphatic filariasis. The elevated levels of filarial γ-GT-specific IgE and IgG1 found in patients with TPE might be relevant for the clearance and destruction of microfilariae in the lungs, a mechanism shown to be very effective in animal models of filariasis (6, 51). Participation of filaria-specific IgE in the killing of microfilariae and/or adult worms is strongly suggested from in vitro experiments (16, 17) and from experimental models of filariasis in cats (1), rats (14), dogs (6), and jirds (45).
Autoimmunity due to molecular mimicry between the parasite γ-GT and human γ-GT present on the surface of the bronchial epithelium and of patients with TPE may also contribute further to the pathologic manifestations of TPE. Humoral autoimmunity to endogenous murine γ-GT on the bronchoalveolar epithelium was demonstrated after immunization of BALB/c mice with recombinant filarial B. malayi γ-GT (13). Autoimmunity to the human enzyme might explain the presence of a persistent lower respiratory tract inflammation associated with interstitial lung disease even after extended antifilarial therapy of patients with TPE (47).
Filarial γ-GT is present in the infectious L3 larvae, a fact that had previously escaped our detection, most probably due to the low concentration of affinity-purified antibodies (29). L3 induces specific IgE in populations in areas of endemic infection (7, 12), allergic responses in exposed individuals (33), transmigrants to areas of endemic infection (42), and exposed military forces (50). In a mouse model of lymphatic filariasis, Brugia L3 was shown to induce a Th2 cytokine profile (39). Thus, the presence of specific IgE to L3 γ-GT in 100% of infection-free ENL patients suggests its involvement in protective immunity. The high IgE/IgG4 ratios in TPE patients is in stark contrast to the much lower ratios in the CP and MF groups; in addition, the elevated IgE/IgG4 ratios in ENL controls suggest the participation of IgE in protective immunity. A similar link was suggested by studies that found an association between (i) high levels of anti-larval IgE and resistance to infection in Schistosoma mansoni-infected populations (46) and (ii) high levels of adult worm-specific IgE and resistance to reinfection after chemotherapy in S. haematobium-infected individuals (9, 10, 15). An association between ABA-1-specific IgE and immunity to Ascaris lumbricoides has recently been reported (34).
Thus, dysregulation in the control of the allergic reactivity toward crucial filarial antigens, as shown here for B. malayi γ-GT, may play an important role in the pathogenesis of pulmonary inflammation. The dichotomy in the IgE antibody function demonstrated in this study agrees with the proposed ontological origin of IgE, being involved not only in protective mechanisms against parasitic infections but also as a potent effector mechanism in allergic diseases (4). Analysis of the immune response to B. malayi γ-GT in longitudinal studies from areas with different endemicity may give insights into the regulation of the IgE and IgG4 responses in infected populations and may therefore elucidate mechanisms of both disease and protective immunity in lymphatic filarial infection of humans.
Acknowledgments
This work was supported by Wellcome Trust grant 052842 and by the Fogarty Foundation, National Institutes of Health, Bethesda, Md.
We thank Annie Higgs for critical reading of the manuscript.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Baldwin, C. I., F. de Medeiros, and D. A. Denham. 1993. IgE responses in cats infected with Brugia pahangi. Parasite Immunol. 15:291-296. [DOI] [PubMed] [Google Scholar]
- 2.Busse, W. W., and R. F. Lemanske, Jr. 2001. Asthma. N. Engl. J. Med. 344:350-362. [DOI] [PubMed] [Google Scholar]
- 3.Capron, A., M. Capron, C. Grangette, and J. P. Dessaint. 1989. IgE and inflammatory cells. Ciba Found. Symp. 147:153-160. [DOI] [PubMed] [Google Scholar]
- 4.Capron, A., J. P. Dessaint, and M. Capron. 1992. Allergy and Immune defense: common IgE-mediated mechanisms or divergent pathways? In R. Moqbel (ed.), Allergy and immunity to helminths. Taylor and Francis Ltd., London, England.
- 5.Capron, M., and A. Capron. 1994. Immunoglobulin E and effector cells in schistosomiasis. Science 264:1876-1877. [DOI] [PubMed] [Google Scholar]
- 6.Castleman, W. L., and M. M. Wong. 1982. Light and electron microscopic pulmonary lesions associated with retained microfilariae in canine occult dirofilariasis. Vet. Pathol. 19:355-364. [DOI] [PubMed] [Google Scholar]
- 7.Das, M. K., M. K. Beuria, and A. P. Dash. 1992. Immunoglobulin E and G4 antibodies to infective larvae in a Wuchereria bancrofti endemic population. Int. Arch. Allergy Immunol. 99:118-122. [DOI] [PubMed] [Google Scholar]
- 8.Del Prete, G. F., M. De Carli, C. Mastromauro, R. Biagiotti, D. Macchia, P. Falagiani, M. Ricci, and S. Romagnani. 1991. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J. Clin. Investig. 88:346-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demeure, C. E., P. Rihet, L. Abel, M. Ouattara, A. Bourgois, and A. J. Dessein. 1993. Resistance to Schistosoma mansoni in humans: influence of the IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J. Infect. Dis. 168:1000-1008. [DOI] [PubMed] [Google Scholar]
- 10.Dunne, D. W., A. E. Butterworth, A. J. Fulford, H. C. Kariuki, J. G. Langley, J. H. Ouma, A. Capron, R. J. Pierce, and R. F. Sturrock. 1992. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur. J. Immunol. 22:1483-1494. [DOI] [PubMed] [Google Scholar]
- 11.Ehigiator, H. N., A. W. Stadnyk, and T. D. Lee. 2000. Extract of Nippostrongylus brasiliensis stimulates polyclonal type-2 immunoglobulin response by inducing de novo class switch. Infect. Immun. 68:4913-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman, D. O., T. B. Nutman, and E. A. Ottesen. 1989. Protective immunity in bancroftian filariasis. Selective recognition of a 43-kD larval stage antigen by infection-free individuals in an endemic area. J. Clin. Investig. 83:14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gounni, A. S., K. Spanel-Borowski, M. Palacios, C. Heusser, S. Moncada, and E. Lobos. 2001. Pulmonary inflammation induced by a recombinant Brugia malayi gamma-glutamyl transpeptidase homolog: involvement of humoral autoimmune responses. Mol. Med. 7:344-354. [PMC free article] [PubMed] [Google Scholar]
- 14.Gusmao, R. D., A. M. Stanley, and E. A. Ottesen. 1981. Brugia pahangi: immunologic evaluation of the differential susceptibility of filarial infection in inbred Lewis rats. Exp. Parasitol. 52:147-159. [DOI] [PubMed] [Google Scholar]
- 15.Hagan, P., U. J. Blumenthal, D. Dunn, A. J. Simpson, and H. A. Wilkins. 1991. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 349:243-245. [DOI] [PubMed] [Google Scholar]
- 16.Haque, A., A. Ouaissi, M. Joseph, M. Capron, and A. Capron. 1981. IgE antibody in eosinophil- and macrophage-mediated in vitro killing of Dipetalonema viteae microfilariae. J. Immunol. 127:716-725. [PubMed] [Google Scholar]
- 17.Haque, A., W. Cuna, B. Bonnel, A. Capron, and M. Joseph. 1985. Platelet mediated killing of larvae from different filarial species in the presence of Dipetalonema viteae stimulated IgE antibodies. Parasite Immunol. 7:517-526. [DOI] [PubMed] [Google Scholar]
- 18.Hussain, R., R. G. Hamilton, V. Kumaraswami, N. F. Adkinson, Jr., and E. A. Ottesen. 1981. IgE responses in human filariasis. I. Quantitation of filaria-specific IgE. J. Immunol. 127:1623-1629. [PubMed] [Google Scholar]
- 19.Hussain, R., and E. A. Ottesen. 1985. IgE responses in human filariasis. III. Specificities of IgE and IgG4 subclass antibodies. J. Immunol. 135:1415-1420. [PubMed] [Google Scholar]
- 20.Hussain, R., and E. A. Ottesen. 1986. IgE responses in human filariasis. IV. Parallel antigen recognition by IgE and IgG antibodies compared by immunoblot analysis. J. Immunol. 136:1859-1863. [PubMed] [Google Scholar]
- 21.Hussain, R., M. Grogl, and E. A. Ottesen. 1987. IgG antibody subclasses in human filariasis. Differential subclass recognition of parasite antigens correlates with different clinical manifestations of infection. J. Immunol. 139:2794-2798. [PubMed] [Google Scholar]
- 22.Hussain, R., R. W. Poindexter, and E. A. Ottesen. 1992. Control of allergic reactivity in human filariasis. Predominant localization of blocking antibody to the IgG4 subclass. J. Immunol. 148:2731-2737. [PubMed] [Google Scholar]
- 23.Kaneko, M., M. C. Swanson, G. J. Gleich, and H. Kita. 1995. Allergen-specific IgG1 and IgG3 through Fc gamma RII induce eosinophil degranulation. J. Clin. Investig. 95:2813-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur, K. J., D. Jain, M. Goel, and D. M. Salunke. 2001. Immunological implications of structural mimicry between a dodecapeptide and a carbohydrate moiety. Vaccine 19:3124-3130. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy, M. W. 2000. The nematode polyprotein allergens/antigens. Parasitol. Today 16:373-380. [DOI] [PubMed] [Google Scholar]
- 26.Kurniawan, A., M. Yazdanbakhsh, R. van Ree, R. Aalberse, M. E. Selkirk, F. Partono, and R. M. Maizels. 1993. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J. Immunol. 150:3941-3950. [PubMed] [Google Scholar]
- 27.Kwan-Lim, G. E., K. P. Forsyth, and R. M. Maizels. 1990. Filarial-specific IgG4 response correlates with active Wuchereria bancrofti infection. J. Immunol. 145:4298-4305. [PubMed] [Google Scholar]
- 28.Lee, T. D., and A. McGibbon. 1993. Potentiation of IgE responses to third-party antigens mediated by Ascaris suum soluble products. Int. Arch. Allergy Immunol. 102:185-190. [DOI] [PubMed] [Google Scholar]
- 29.Lobos, E., A. Ondo, E. A. Ottesen, and T. B. Nutman. 1992. Biochemical and immunologic characterization of a major IgE-inducing filarial antigen of Brugia malayi and implications for the pathogenesis of tropical pulmonary eosinophilia. J. Immunol. 149:3029-3034. [PubMed] [Google Scholar]
- 30.Lobos, E., R. Zahn, N. Weiss, and T. B. Nutman. 1996. A major allergen of lymphatic filarial nematodes is a parasite homolog of the gamma-glutamyl transpeptidase. Mol. Med. 2:712-724. [PMC free article] [PubMed] [Google Scholar]
- 31.Lobos, E. 1997. The basis of IgE responses to specific antigenic determinants in helminthiasis. Chem. Immunol. 66:1-25. [DOI] [PubMed] [Google Scholar]
- 32.Marley, S. E., P. J. Lammie, M. L. Eberhard, and A. W. Hightower. 1995. Reduced antifilarial IgG4 responsiveness in a subpopulation of microfilaremic persons. J. Infect. Dis. 172:1630-1633. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy, J. S., E. A. Ottesen, and T. B. Nutman. 1994. Onchocerciasis in endemic and nonendemic populations: differences in clinical presentation and immunologic findings. J. Infect. Dis. 170:736-741. [DOI] [PubMed] [Google Scholar]
- 34.McSharry, C., Y. Xia, C. V. Holland, and M. W. Kennedy. 1999. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect. Immun. 67:484-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.More, S. J., and D. B. Copeman. 1990. A highly specific and sensitive monoclonal antibody-based ELISA for the detection of circulating antigen in bancroftian filariasis. Trop. Med. Parasitol. 41:403-406. [PubMed] [Google Scholar]
- 36.Nutman, T. B., V. Kumaraswami, L. Pao, P. R. Narayanan, and E. A. Ottesen. 1987. An analysis of in vitro B cell immune responsiveness in human lymphatic filariasis. J. Immunol. 138:3954-3959. [PubMed] [Google Scholar]
- 37.Nutman, T. B., V. K. Vijayan, P. Pinkston, V. Kumaraswami, C. Steel, R. G. Crystal, and E. A. Ottesen. 1989. Tropical pulmonary eosinophilia: analysis of antifilarial antibody localized to the lung. J. Infect. Dis. 160:1042-1050. [DOI] [PubMed] [Google Scholar]
- 38.Nutman, T. B., and V. Kumaraswami. 2001. Regulation of the immune response in lymphatic filariasis: perspectives on acute and chronic infection with Wuchereria bancrofti in South India. Parasite Immunol. 23:389-399. [DOI] [PubMed] [Google Scholar]
- 39.Osborne, J., and E. Devaney. 1998. The L3 of Brugia induces a Th2-polarized response following activation of an IL-4-producing CD4−CD8− alphabeta T cell population. Int. Immunol. 10:1583-1590. [DOI] [PubMed] [Google Scholar]
- 40.Ottesen, E. A., F. Skvaril, S. P. Tripathy, R. W. Poindexter, and R. Hussain. 1985. Prominence of IgG4 in the IgG antibody response to human filariasis. J. Immunol. 134:2707-2712. [PubMed] [Google Scholar]
- 41.Ottesen, E. A., and T. B. Nutman. 1992. Tropical pulmonary eosinophilia. Annu. Rev. Med. 43:417-424. [DOI] [PubMed] [Google Scholar]
- 42.Partono, F., P. W. Pribadi, and A. Soewarta. 1978. Epidemiological and clinical features of Brugia timori in a newly established village, Karakuak, West Flores, Indonesia. Am. J. Trop. Med. Hyg. 27:910-915. [DOI] [PubMed] [Google Scholar]
- 43.Paxton, W. A., M. Yazdanbakhsh, A. Kurniawan, F. Partono, R. M. Maizels, and M. E. Selkirk. 1993. Primary structure of and immunoglobulin E response to the repeat subunit of gp15/400 from human lymphatic filarial parasites. Infect. Immun. 61:2827-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearlman, E., F. E. Hazlett, Jr., W. H. Boom, and J. W. Kazura. 1993. Induction of murine T-helper-cell responses to the filarial nematode Brugia malayi. Infect. Immun. 61:1105-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao, U. R., C. Nasarre, S. U. Coleman, M. Bakeer, V. A. Dennis, D. W. Horohov, and T. R. Klei. 1996. Cellular immune responses of jirds to extracts of life cycle stages and adult excretory secretory products during the early development of Brugia pahangi. Exp. Parasitol. 82:255-266. [DOI] [PubMed] [Google Scholar]
- 46.Rihet, P., C. E. Demeure, A. Bourgois, A. Prata, and A. J. Dessein. 1991. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur. J. Immunol. 21:2679-2686. [DOI] [PubMed] [Google Scholar]
- 47.Rom, W. N., V. K. Vijayan, M. J. Cornelius, V. Kumaraswami, R. Prabhakar, E. A. Ottesen, and R. G. Crystal. 1990. Persistent lower respiratory tract inflammation associated with interstitial lung disease in patients with tropical pulmonary eosinophilia following conventional treatment with diethylcarbamazine. Am. Rev. Respir. Dis. 142:1088-1092. [DOI] [PubMed] [Google Scholar]
- 48.Schur, P. H. 1987. IgG subclasses. A review. Ann. Allergy 58:89-97. [PubMed] [Google Scholar]
- 49.Stromberg, B. E. 1979. IgE and IgG1 antibody production by a soluble product of Ascaris suum in the guinea-pig. Immunology 38:489-495. [PMC free article] [PubMed] [Google Scholar]
- 50.Wartman, W. B. 1947. Filariasis in American armed forces in World War II. Medicine 26:333-394. [DOI] [PubMed] [Google Scholar]
- 51.Weil, G. J., K. G. Powers, E. L. Parbuoni, B. R. Line, R. D. Furrow, and E. A. Ottesen. 1982. Dirofilaria immitis. VI. Antimicrofilarial immunity in experimental filariasis. Am. J. Trop. Med. Hyg. 31:477-485. [PubMed] [Google Scholar]
- 52.Willers, J., A. Lucchese, D. Kanduc, and S. Ferrone. 1999. Molecular mimicry of phage displayed peptides mimicking GD3 ganglioside. Peptides 20:1021-1026. [DOI] [PubMed] [Google Scholar]
- 53.Yazdanbakhsh, M., W. A. Paxton, Y. C. Kruize, E. Sartono, A. Kurniawan, A. van het Wout, M. E. Selkirk, F. Partono, and R. M. Maizels. 1993. T cell responsiveness correlates differentially with antibody isotype levels in clinical and asymptomatic filariasis. J. Infect. Dis. 167:925-931. [DOI] [PubMed] [Google Scholar]
- 54.Yazdanbakhsh, M., W. A. Paxton, A. Brandenburg, R. van Ree, M. Lens, F. Partono, R. M. Maizels, and M. E. Selkirk. 1995. Differential antibody isotype reactivity to specific antigens in human lymphatic filariasis: gp15/400 preferentially induces immunoglobulin E (IgE), IgG4, and IgG2. Infect. Immun. 63:3772-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yazdanbakhsh, M., A. van den Biggelaar, and R. M. Maizels. 2001. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol. 22:372-377. [DOI] [PubMed] [Google Scholar]