Abstract
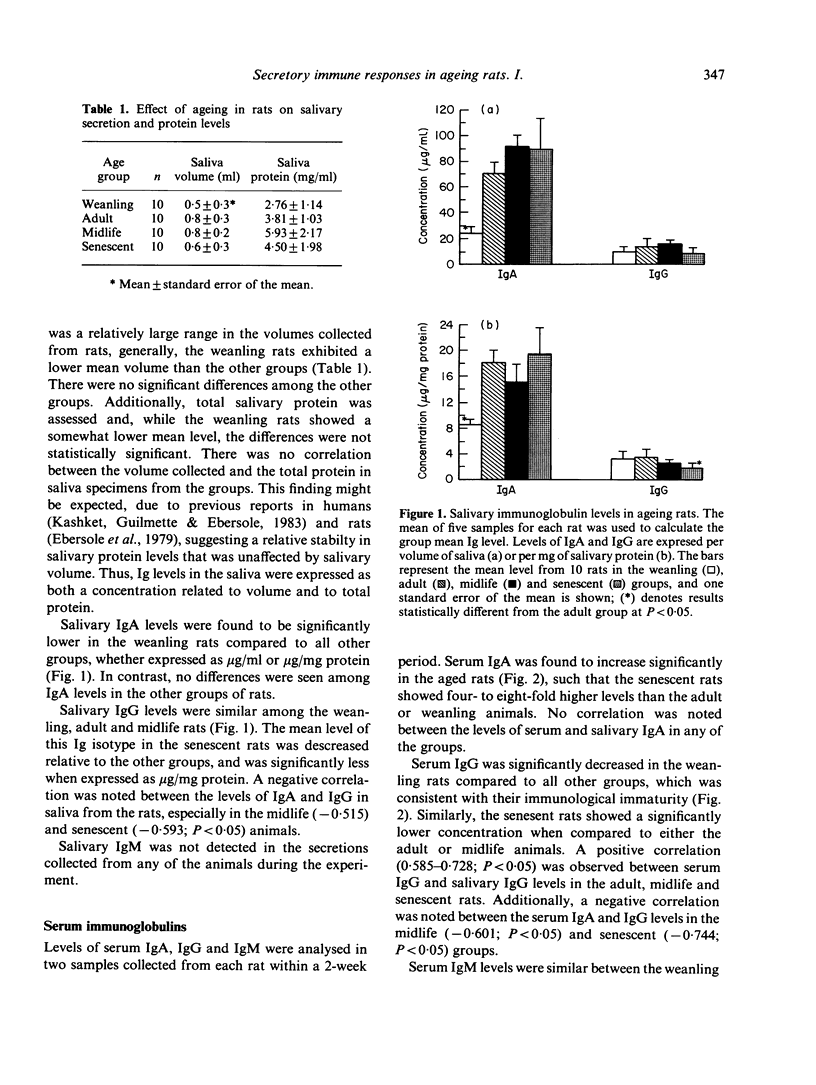
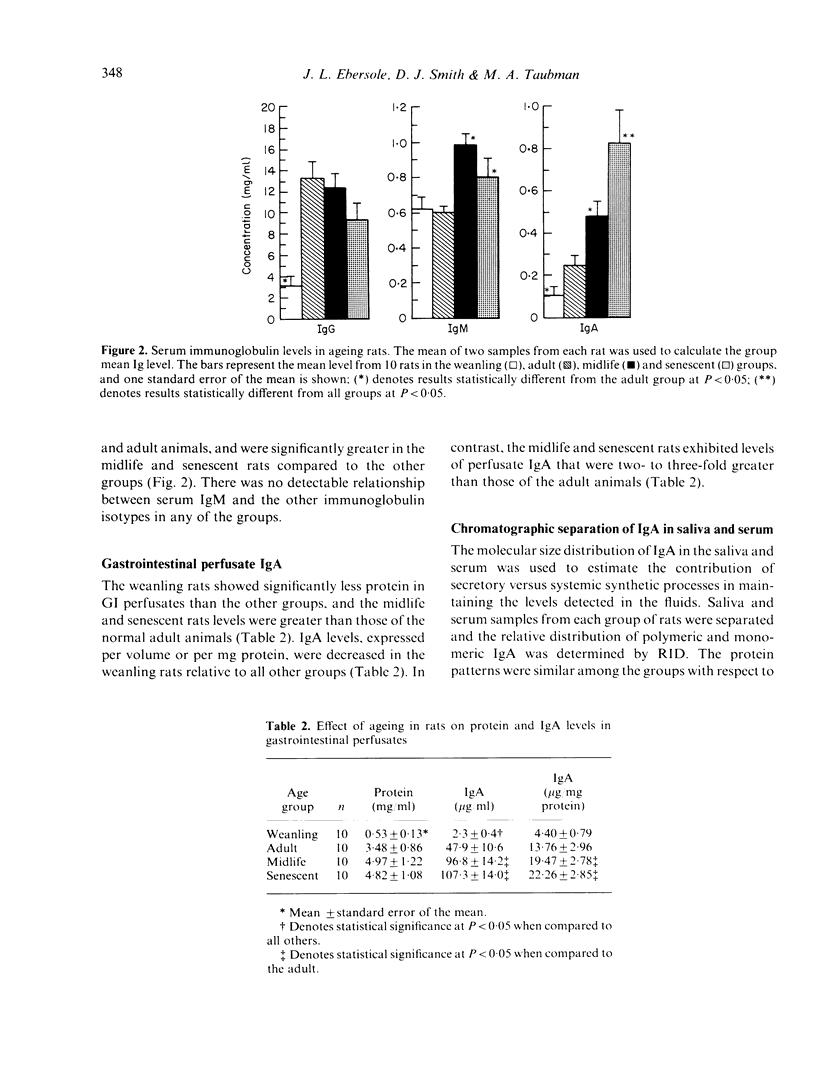
Immunoglobulin levels in saliva, serum and gastrointestinal perfusates were measured in groups of ageing CDF (F-344)Cr1BR rats. Four age groups were studied, including: (i) weanling (21-35 days), (ii) adult (3-4 months), (iii) midlife (10-12 months), and (iv) senescent (18-20 months). There was no difference in the mean salivary volume and protein levels in the three older groups of rats. Salivary IgA in the weanling rats was significantly lower, having not yet attained adult levels, while salivary IgG was decreased in the senescent group. No IgM was detected in saliva from any of the animals. Serum IgM was elevated in the midlife and senescent rats. In contrast, serum IgG was significantly decreased in the senescent group when compared to the adult or midlife animals. Significant elevations were noted in serum and intestinal perfusate IgA in the senescent rats when compared to the adult group. While salivary IgA from all groups was shown to be greater than 95% polymeric, only the weanling and senescent groups exhibited a tendency toward predominantly polymeric serum IgA (67-70%). The results define altered immunoglobulin profiles in aged rats in both secretory and systemic fluids, which could affect the immunocompetence of these animals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford R. H. Effects of chronic bronchopulmonary disease and aging on human nasal secretion IgA concentrations. J Immunol. 1968 Nov;101(5):984–988. [PubMed] [Google Scholar]
- Cypess R. H., Ebersole J. L., Molinari J. A. Specific antibody levels in the intestinal perfusates of Heligosomoides polygyrus-infected mice. Int Arch Allergy Appl Immunol. 1977;55(1-6):496–503. doi: 10.1159/000231963. [DOI] [PubMed] [Google Scholar]
- Ebersole J. L., Taubman M. A., Smith D. J., Frey D. E. Effect of neonatal thymectomy on immune responses of rats to Streptococcus mutans. Infect Immun. 1982 Sep;37(3):993–1000. doi: 10.1128/iai.37.3.993-1000.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole J. L., Taubman M. A., Smith D. J. The effect of neonatal thymectomy on the level of salivary and serum immunoglobulins in rats. Immunology. 1979 Apr;36(4):649–657. [PMC free article] [PubMed] [Google Scholar]
- Haaijman J. J., Schuit H. R., Hijmans W. Immunoglobulin-containing cells in different lymphoid organs of the CBA mouse during its life-span. Immunology. 1977 Apr;32(4):427–434. [PMC free article] [PubMed] [Google Scholar]
- Kishimoto S., Tomino S., Inomata K., Kotegawa S., Saito T., Kuroki M., Mitsuya H., Hisamitsu S. Age-related changes in the subsets and functions of human T lymphocytes. J Immunol. 1978 Nov;121(5):1773–1780. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Makinodan T., Kay M. M. Age influence on the immune system. Adv Immunol. 1980;29:287–330. doi: 10.1016/s0065-2776(08)60047-4. [DOI] [PubMed] [Google Scholar]
- Nordin A. A., Makinodan T. Humoral immunity in aging. Fed Proc. 1974 Sep;33(9):2033–2035. [PubMed] [Google Scholar]
- Orlans E., Peppard J., Reynolds J., Hall J. Rapid active transport of immunoglobulin A from blood to bile. J Exp Med. 1978 Feb 1;147(2):588–592. doi: 10.1084/jem.147.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sacci J. B., Jr, Craig J. P., Germanier R., Fürer E. Oral immunization against experimental cholera: the role of antigen form and antigen combinations in evoking protection. Ann N Y Acad Sci. 1983 Jun 30;409:724–733. doi: 10.1111/j.1749-6632.1983.tb26911.x. [DOI] [PubMed] [Google Scholar]
- Rivier D. A., Trefts P. E., Kagnoff M. F. Age-dependence of the IgA anti-alpha (1 leads to 3) dextran B1355 response in vitro. Scand J Immunol. 1983 Feb;17(2):115–121. doi: 10.1111/j.1365-3083.1983.tb00773.x. [DOI] [PubMed] [Google Scholar]
- Schwartz A. R., Togo Y., Hornick R. B. Clinical evaluation of live, oral types 1, 2, and 5 adenovirus vaccines. Am Rev Respir Dis. 1974 Feb;109(2):233–239. doi: 10.1164/arrd.1974.109.2.233. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Ebersole J. L., Taubman M. A. Local and systemic immune response in aged hamsters. Immunology. 1983 Nov;50(3):407–413. [PMC free article] [PubMed] [Google Scholar]
- Szewczuk M. R., Campbell R. J., Jung L. K. Lack of age-associated immune dysfunction in mucosal-associated lymph nodes. J Immunol. 1981 Jun;126(6):2200–2204. [PubMed] [Google Scholar]
- Taubman M. A., Buckelew J. M., Ebersole J. L., Smith D. J. Periodontal bone loss and immune response to ovalbumin in germfree rats fed antigen-free diet with ovalbumin. Infect Immun. 1981 Apr;32(1):145–152. doi: 10.1128/iai.32.1.145-152.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


