Abstract
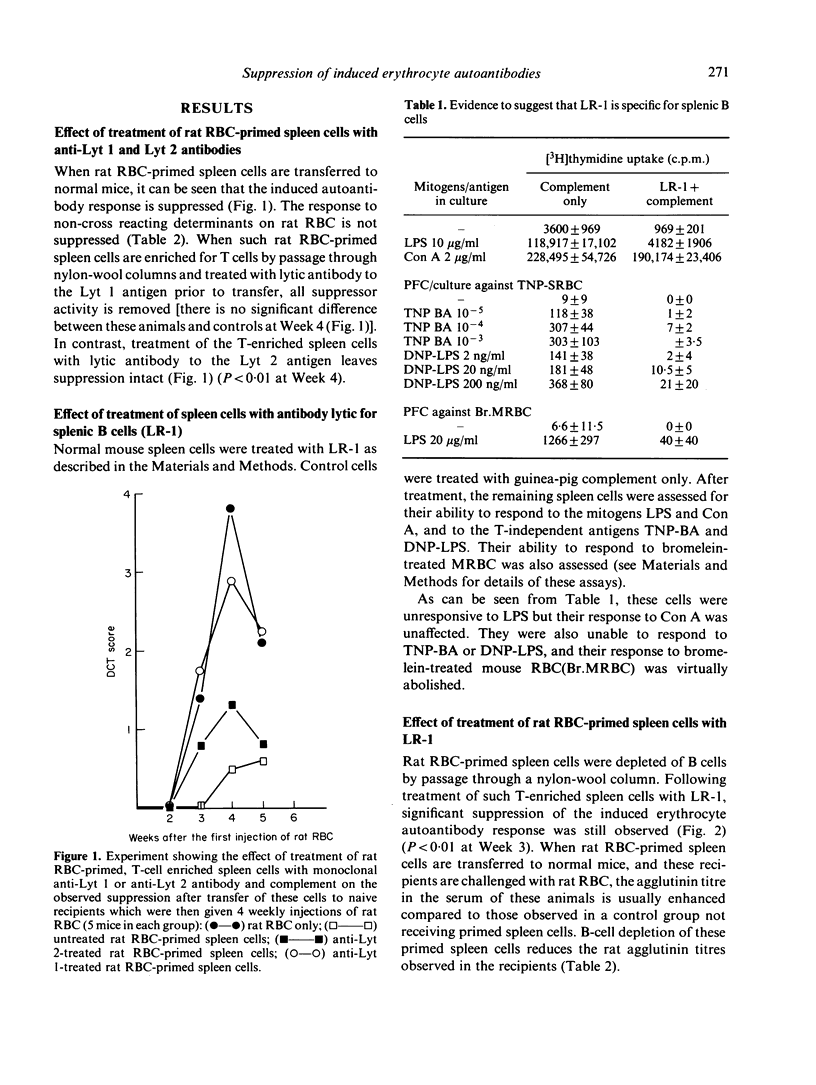
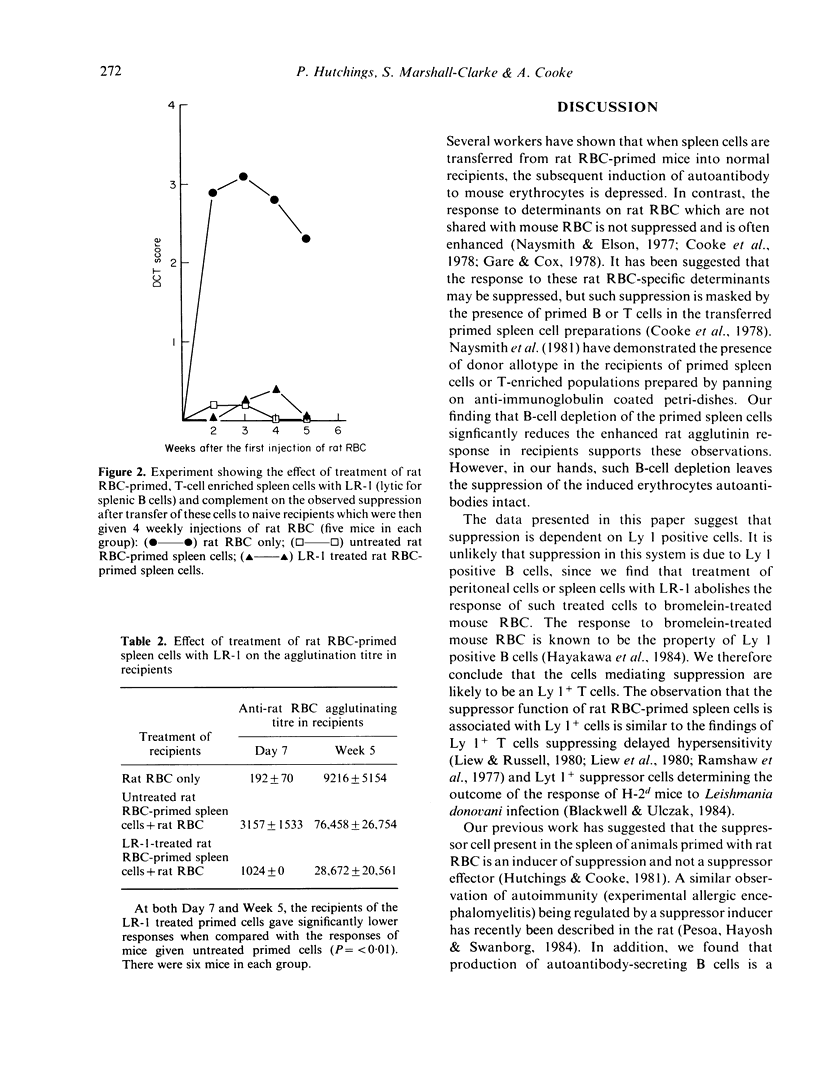
When normal mice are immunized with rat RBC, autoantibodies to mouse red cells and antigen-specific suppressor cells are generated. Suppressor cell activity is found in T-enriched populations and suppresses only the induction of autoantibody, not ongoing or secondary autoantibody responses. Using antibodies lytic for either B cells or distinct T-cell subpopulations, we are able to show that suppression is dependent on the presence of Lyt 1+ T cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell J. M., Ulczak O. M. Immunoregulation of genetically controlled acquired responses to Leishmania donovani infection in mice: demonstration and characterization of suppressor T cells in noncure mice. Infect Immun. 1984 Apr;44(1):97–102. doi: 10.1128/iai.44.1.97-102.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayen A., Parkhouse R. M. Preparation and properties of a cytotoxic monoclonal rat anti-mouse Thy-1 antibody. J Immunol Methods. 1982;49(1):17–23. doi: 10.1016/0022-1759(82)90362-3. [DOI] [PubMed] [Google Scholar]
- Cooke A., Hutchings P. R., Playfair J. H. Suppressor T cells in experimental autoimmune haemolytic anaemia. Nature. 1978 May 11;273(5658):154–155. doi: 10.1038/273154a0. [DOI] [PubMed] [Google Scholar]
- Cooke A., Hutchings P. Defective regulation of erythrocyte autoantibodies in SJL mice. Immunology. 1984 Mar;51(3):489–492. [PMC free article] [PubMed] [Google Scholar]
- Cooke A., Hutchings P., Marshall-Clarke S. Lack of autoantigen-specific splenic suppressor cells in mice with an X-linked B-lymphocyte defect. Immunology. 1980 Dec;41(4):815–818. [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J. Large numbers of cells in normal mice produce antibody components of isologous erythrocytes. Nature. 1974 Dec 20;252(5485):749–751. doi: 10.1038/252749a0. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C., Wright B. Rat x rat hybrid myelomas and a monoclonal anti-Fd portion of mouse IgG. Nature. 1979 Jan 11;277(5692):131–133. doi: 10.1038/277131a0. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings P., Cooke A. Analysis of the cellular interactions involved in the regulation of induced erythrocyte autoantibodies. Cell Immunol. 1981 Sep 15;63(2):221–227. doi: 10.1016/0008-8749(81)90001-0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Sia D. Y., Parish C. R., McKenzie I. F. Major histocompatibility gene complex (MHC)-coded determinants on antigen-specific suppressor factor for delayed-type hypersensitivity and surface phenotypes of cells producing the factor. Eur J Immunol. 1980 Apr;10(4):305–309. doi: 10.1002/eji.1830100415. [DOI] [PubMed] [Google Scholar]
- Mond J. J., Scher I., Mosier D. E., Baese M., Paul W. E. T-independent responses in B cell-defective CBA/N mice to Brucella abortus and to trinitrophenyl (TNP) conjugates of Brucella abortus. Eur J Immunol. 1978 Jul;8(7):459–463. doi: 10.1002/eji.1830080703. [DOI] [PubMed] [Google Scholar]
- Naysmith J. D., Ortega-Pierres M. G., Elson C. J. Rat erythrocyte-induced anti-erythrocyte autoantibody production and control in normal mice. Immunol Rev. 1981;55:55–87. doi: 10.1111/j.1600-065x.1981.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Pesoa S. A., Hayosh N. S., Swanborg R. H. Regulation of experimental allergic encephalomyelitis. Part 5. Role of the recipient in suppressor cell induction. J Neuroimmunol. 1984 Dec;7(2-3):131–135. doi: 10.1016/s0165-5728(84)80013-2. [DOI] [PubMed] [Google Scholar]
- Ramshaw I. A., McKenzie I. F., Bretscher P. A., Parish C. R. Discrimination of suppressor T cells of humoral and cell-mediated immunity by anti-Ly and anti-Ia sera. Cell Immunol. 1977 Jun 15;31(2):364–369. doi: 10.1016/0008-8749(77)90038-7. [DOI] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]



