Abstract
Previous studies with mice have shown that major histocompatibility complex class II (MHC-II) is required for protection from Helicobacter pylori, while MHC-I and antibodies are not. Thus, CD4+ T cells are presumed to play an essential role in protective immunity via secretion of cytokines. To determine which cytokines are associated with a reduction of bacterial load in immunized mice, gastric cytokine expression was examined by semiquantitative reverse transcription-PCR in protected (defined as ≥2-log-unit decrease in bacterial load) and unprotected mice 4 weeks after challenge. Elevated levels of mRNA for interleukin-12p40 (IL-12p40), gamma interferon (IFN-γ), tumor necrosis factor alpha, and inducible nitric oxide synthase (iNOS) were associated with protection in immunized-challenged (I/C) mice, but Th2 cytokine (IL-4, IL-5, IL-10, and IL-13) and chemokine (KC, MIP-2, and MCP-1) expression was not associated with protection. Despite the association of IFN-γ and iNOS message with protection, I/C mice genetically lacking either of these products were able to reduce the bacterial load as well as the wild-type I/C controls. The I/C mice lacking IL-12p40 were not protected compared to unimmunized-challenged mice. All I/C groups developed gastritis. We conclude that neither IFN-γ nor iNOS is essential for vaccine-induced protection from H. pylori infection. The p40 subunit of IL-12, which is a component of both IL-12 and IL-23, is necessary for protection in immunized mice. These findings suggest a novel IFN-γ-independent function of IL-12p40 in effective mucosal immunization against H. pylori.
Infection with Helicobacter pylori results in antibody production and an inflammatory infiltrate of neutrophils, lymphocytes, plasma cells, macrophages, and eosinophils in the gastric mucosa. In spite of this immune response, H. pylori infection in most untreated people is lifelong. Mice can be protected from H. pylori infection by immunization, and based on the lack of protection in major histocompatibility complex class II-deficient immunized mice, the protection is presumed to require CD4+ T cells (9, 15, 26, 29, 33). The need for CD4+ T cells suggests that the cytokines they secrete play a role in immunity.
Numerous studies of biopsies from H. pylori-infected patients have demonstrated proinflammatory cytokines and chemokines such as gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-2, IL-6, IL-12, IL-8, MIP-1α, and GROα in the gastric mucosa (6, 19, 31, 36, 45-47). The methods, i.e., reverse transcription-PCR (RT-PCR) and enzyme-linked immunosorbent assay, used in these studies to detect cytokines and chemokines do not allow discrimination of which cells are producing the mediators. However, immunohistochemistry of biopsies has revealed cytokine-containing cells in the epithelium and lamina propria. Increased IL-8 immunofluorescence was observed in the epithelia from patients with H. pylori-induced gastritis compared to normal gastric epithelium (7). Also, greater numbers of epithelial cells, intraepithelial lymphocytes, and lamina propria cells positive for IL-1β, IL-6, IL-8, and TNF-α were found in H. pylori-infected patients than in uninfected individuals (24). T cells isolated from biopsies of infected patients produced IL-2, IFN-γ, and TNF-α but rarely Th2 cytokines (IL-4 and IL-5), leading to the conclusion that T cells in the infected gastric mucosa have a Th1 phenotype (3, 39). In contrast, one study found an increase in CD8+ T cells and NK cells by fluorescence-activated cell sorter analysis of isolated gastric lymphocytes from infected patients and an increase of IL-4 and IL-6 in supernatants of cultured antral biopsies (1). Finally, H. pylori-specific T-cell clones generated from biopsies of infected patients expressed mainly IFN-γ (20 of 24 clones tested) or a combination of IFN-γ, IL-4, and IL-5 (4 of 24 clones) when stimulated with H. pylori antigens (8). TNF-α was secreted by all the H. pylori-reactive clones. Overall, the preponderance of proinflammatory cytokines and Th1 T cells has lead to the idea that H. pylori-induced gastritis is a Th1-mediated disease (16, 25).
Much less is known about cytokines and chemokines in the gastric mucosa of H. pylori-infected or immunized mice. High levels of IFN-γ were constitutively produced by lymphocytes isolated from the gastric lamina propria of Helicobacter felis-infected or immunized-challenged (I/C) C57BL/6 mice (28). mRNA expression for IFN-γ, TNF-α, IL-1β, RANTES, MIP-2, IP-10, and MCP-1 was detected in C57BL/6 mice infected with H. felis (11). Immunized mice protected from H. pylori had relatively high levels of mRNA for IFN-γ but undetectable to low levels of IL-4 and IL-5 (14). In situ hybridization for IFN-γ and IL-4 mRNAs in C57BL/6 mice demonstrated a greater prevalence of IFN-γ-expressing cells in H. pylori-infected mice than in naive controls but no difference in IL-4-positive cells (38). Taken together these results suggest that IFN-γ is produced in the gastric mucosa of mice in response to Helicobacter sp. and may be related to gastritis and/or protection.
To determine which cytokines are associated with protection from H. pylori infection, gastric samples from protected and unprotected I/C mice, unimmunized-not challenged (U/NC) mice, and unimmunized-challenged (U/C) mice were screened for a panel of cytokines and chemokines. Elevated levels of IL-12p40, IFN-γ, TNF-α, and inducible nitric oxide synthase (iNOS) mRNAs appeared to be associated with protection, and the biologic effects of IL-12p40, IFN-γ, and iNOS were studied in mice genetically deficient in these products.
MATERIALS AND METHODS
Mice.
Female 5- to 6-week-old, specific-pathogen-free, Helicobacter species-negative C57BL/6 mice were purchased from Harlan (Indianapolis, Ind.) and from The Jackson Laboratory (TJL) (Bar Harbor, Maine). Twenty-four female C57BL/6J-Nos2tm1Lau (stock no. 002609) mice were purchased from TJL. Three pairs of C57BL/6J breeders were purchased from TJL in order to produce control mice for some experiments. Mice deficient in IFN-γ and IL-12p40 were bred from C57BL/6J-Ifngtm1Ts (stock no. 002287), and C57BL/6J-Il12btm1Jm (stock no. 002693) breeders previously obtained from TJL. All mice were housed in autoclaved microisolators and provided with autoclaved water and sterilized Teclad chow ad libitum. All procedures involving mice were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University.
Bacteria and bacterial products.
H. pylori SS1 (23) which had been passaged through a BALB/c mouse was donated by Steven Danon of Ohio State University, Columbus, and cultured as described recently (12). Briefly, H. pylori was grown at 37°C on Columbia blood agar plates containing 7% horse blood (Cleveland Scientific, Columbus, Ohio) and 2.5 μg of amphotericin B (Sigma, St. Louis, Mo.) per ml in a microaerobic high-CO2 atmosphere. For isolation of H. pylori from stomachs, the plates also contained bacitracin (200 μg/ml), vancomycin (6 μg/ml), cefsulodin (16 μg/ml), and trimethoprim (20 μg/ml) (Sigma) to inhibit the growth of gastric flora. For challenge, H. pylori was grown in static liquid cultures of brucella broth (Difco, Sparks, Md.), 10.5% fetal bovine serum (GibcoBRL, Frederick, Md.), amphotericin (3 μg/ml), vancomycin (7.5 μg/ml), cefsulodin (20 μg/ml), and trimethoprim (25 μg/ml). The liquid culture was passaged at least once but not more than five times before inoculation of mice. Growth curves have shown that an increase in absorbance of 0.09 to 0.1 above that of uninoculated medium at 450 nm gives the highest viable count (2 × 107 to 7 × 107 CFU/ml) in the log growth phase. For immunization, two antigen preparations of H. pylori were prepared, one from plate-cultured bacteria (P antigen) and the other from liquid cultures (L antigen). H. pylori was harvested from plates in 1 ml of sterile Dulbecco's phosphate-buffered saline (GibcoBRL)/plate after 3 to 6 days of culture, pooled, and stored at −80° before sonication. Bacteria grown in liquid culture were harvested after 3 to 6 days when a static growth plateau had been reached and were pelleted by centrifugation at 5,000 × g for 20 min. The pellets were resuspended in sterile phosphate-buffered saline and stored at −80°C prior to sonication. Frozen suspensions were thawed and sonicated on ice eight times for 30-s intervals (continuous power 5 at 50% duty cycle) with a Vibra Cell sonicator (Sonics and Materials, Newtown, Conn.). The 5,000 × g supernatants of the sonicates were filtered through 0.45-μm-pore-size syringe filters and stored at −80°C. The protein concentration of each sonicate was determined by a Lowry assay.
Experimental design.
Six immunization-challenge experiments were performed as described below; three of these included adjuvant control (Adj/C) groups. In each experiment, mice were immunized once a week for 4 weeks, challenged 2 weeks after the last immunization by gavage with 0.5 ml of H. pylori liquid culture containing 1.4 × 107 to 4.1 × 107 CFU (confirmed by quantitative culture), and sacrificed 4 weeks after challenge. A longitudinal strip of glandular stomach approximately 3 to 5 mm wide was cut aseptically from the greater curvature of the stomach and fixed in buffered 10% formalin for histology. The remaining glandular stomach was divided longitudinally along the lesser curvature. One piece of stomach was frozen in liquid nitrogen for RNA isolation; the other was homogenized in 200 μl of brucella broth, serially diluted, and plated to assess colonization (12). Colonies were counted after 5 or 6 days of incubation, and representative colonies were subcultured, Gram stained, and tested for urease, oxidase, and catalase activities to confirm their identity. Bacterial load is expressed as log CFU per gram of stomach. Challenged mice which were culture negative were assigned a value of 1 CFU per gram in order to calculate log CFU per gram and geometric means. Negative control mice, (U/NC mice), were gavaged with sterile brucella broth and cultured as described above. All U/NC mice were culture negative for H. pylori and are not shown in colonization figures.
(i) Antigen-route experiment.
The first experiment compared the two antigen preparations and two routes of antigen administration. There were six treatment groups of six female Harlan C57BL/6 mice per group, including a U/NC group, four I/C groups, and a U/C group. Two I/C groups were immunized intragastrically, one with P antigen and the other with L antigen. For intragastric immunization, antigen was mixed with cholera toxin (CT) (List Biological Laboratories, Inc., Campbell, Calif.), 1 M NaHCO3, and water to produce a suspension of 2 mg of antigen and 10 μg of CT per 0.5 ml in 0.2 M NaHCO3. Each mouse received 0.5 ml by gavage, with care taken to avoid regurgitation. The other two I/C groups were immunized intranasally with 20 μl of a mixture containing 100 μg of P or L antigen and 5 μg of CT diluted in water. The antigen mixture was applied to the external nares of unanesthetized mice in 5-μl aliquots. Technically, this is intranasal-oral administration because some of the antigen runs down the philtrum into the mouth. Based on the results of the antigen-route experiment, intranasal immunization with 100 μg of the P antigen and 5 μg of CT was used in all subsequent experiments.
(ii) iNOS experiment.
To determine whether iNOS is important in clearance of H. pylori infection, female C57BL/6J-Nos2tm1Lau (iNOS knockout [KO]) and C57BL/6J (wild type [WT]) mice were immunized and challenged. For both strains, n was 6 for the U/NC groups and 9 for the U/C groups. There were nine I/C iNOS KO and eight I/C WT mice.
(iii) IL-12 and IFN-γ experiment 1.
Experiments were performed to determine whether IFN-γ or IL-12 is required for protection. The C57BL/6J-Ifngtm1Ts (IFN-γ KO), and C57BL/6J-Il12btm1Jm (IL-12 KO) mice were bred in our animal facility, and both male and female mice were used. For experiment 1, the WT female C57BL/6 mice were from Harlan. The number of mice per treatment group is shown in Table 1. An Adj/C group of WT mice was immunized with a mixture of 100 μg of ovalbumin (grade V; Sigma) and 5 μg of CT.
TABLE 1.
Numbers of animals used for experiments with IFN-γ- or IL-12p40-deficient mice
| Groupa | No. of mice/group in expt:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1
|
2 (IFN-γ)
|
2 (IL-12)
|
3
|
||||||
| IFN-γb | IL-12c | WTd | IFN-γ | WT | IL-12 | WT | IL-12 | WT | |
| U/NC | 4 | 5 | 3 | 3 | 3 | 5 | 3 | 4 | 4 |
| I/C | 4 | 6 | 6 | 9 | 9 | 9 | 8 | 9 | 9 |
| Adj/C | NDe | ND | 5 | 6 | 6 | 7 | 6 | ND | ND |
| U/C | 4 | 6 | 6 | 6 | 6 | 8 | 6 | 9 | 9 |
I/C groups were immunized intranasally four times with 100 μg of sonicate and 5 μg of CT, and Adj/C groups were immunized intranasally four times with 100 μg of ovalbumin and 5 μg of CT. At 2 weeks after the final immunization, U/NC mice received 0.5 ml of sterile broth by gavage, while immunized and U/C groups received 107 CFU of H. pylori SS1 in broth by gavage.
Mice lacking IFN-γ (C57BL/6J-Ifngtm1Ts).
Mice lacking IL-12p40 (C57BL/6J-Il12btm1Jm).
The background strain (C57BL/6).
ND, not done.
(iv) IFN-γ KO experiment 2.
A second experiment using a larger number of IFN-γ KO mice and including Adj/C immunizations in both strains was performed (Table 1). The WT mice were from TJL.
(v) IL-12 KO experiments 2 and 3.
Two additional experiments compared protection in WT and IL-12 KO mice. The WT mice were C57BL/6J. The second experiment included Adj/C groups for both strains (Table 1).
RNA isolation.
RNA was isolated from segments of glandular stomach that had been snap frozen in liquid nitrogen and stored at −80°C by using RNA STAT-60 (Tel-Test, Friendswood, Tex.) with slight modifications to the manufacturer's instructions. Stomachs were homogenized in 1.5-ml disposable polypropylene tissue grinders (Kontes, Vineland, N.J.) in 100 μl of RNA STAT-60 on ice. An additional 100 μl was added, and homogenization was repeated. After addition of 800 μl of RNA STAT-60, the tubes were vortexed and incubated at room temperature for 5 min. Chloroform (200 μl) was added, and tubes were vortexed for 15 s and placed on ice for 12 min. Phases were separated by centrifugation for 20 min at 12,000 × g in a refrigerated microcentrifuge. The upper aqueous phase was removed to a new 1.5-ml tube, and RNA was precipitated by addition of 0.5 ml of isopropanol. The tubes were allowed to stand at −20°C for at least 12 h. The precipitate was pelleted by centrifugation for 15 min at 12,000 × g at 4°C, and the pellets were washed with 1 ml of 75% ethanol. After removal of the supernatant, the pellets were dried at room temperature for 5 min before resuspension in 100 μl diethyl pyrocarbonate-treated water. The RNA concentration was determined by the absorbance at 260 nm. Twenty units of recombinant RNase inhibitor (GibcoBRL) was added, and RNA was stored at −80°C.
RT-PCR.
Three micrograms of total RNA was reverse transcribed with oligo(dT)12-18 primer (GibcoBRL) and RNase H− reverse transcriptase (GibcoBRL) at 42°C overnight. Each 20-μl reaction mixture contained 0.5 μg of primer, first-strand buffer (final concentrations of 50 mM Tris-HCl [pH 8.3], 75 mM KCl, and 3 mM MgCl2), 10 mM dithiothreitol, deoxynucleoside triphosphates (0.5 mM each), 40 U of recombinant RNase inhibitor, and 200 U of reverse transcriptase. After incubation at 42°C, tubes were heated to 70°C for 15 min and stored at −20°C. For each PCR, 10% of the RT reaction mixture was amplified with primer pairs specific for mice (Table 2). Primers for IL-4, IL-5, IFN-γ, and hypoxanthine phosphoribosyl transferase (HPRT) were synthesized by GibcoBRL; all other primers were made by IDT (Coralville, Iowa). PCRs with 50-μl reaction mixtures were carried out in a Perkin-Elmer Cetus thermal cycler, using 2.5 U of Taq DNA polymerase complexed to an inhibitor of polymerase activity (GibcoBRL) with the 10× PCR buffer and MgCl2 solutions at final concentrations of 20 mM Tris-HCl (pH 8.4), 50 mM KCl, and 1.5 mM MgCl2, deoxynucleoside triphosphates (GibcoBRL) at 0.2 mM each, and primers at 0.2 μM each. After a 3-min activation period at 94°C, 35 cycles (for each cytokine, chemokine, and iNOS) of 1 min at 94°C, 1 min 15 s at 55°C, and 1 min 15 s at 72°C were followed by a 7-min final extension at 72°C. The housekeeping gene product HPRT was amplified for 25 cycles. The products were separated on 1.5% agarose gels containing 0.5 μg of ethidium bromide per ml in TAE buffer (0.04 M Tris-acetate, 1 mM EDTA). Products were visualized and photographed with short-wave UV light. All stomachs from the antigen-route experiment were analyzed for IFN-γ, IL-4, IL-5, iNOS, and HPRT. Half of the samples were also screened for IL-10, IL-12p40, IL-13, TNF-α, transforming growth factor β2 (TGF-β2), KC, MIP-1α, MIP-2, and MCP-1. To screen for the larger panel of cytokines, two RT reactions were performed for each sample and the cDNAs were combined so that the same cDNA sample was analyzed for all of the cytokines in each mouse. To obtain more quantitative results for the iNOS product, the iNOS and HPRT agarose gels were scanned with a FluorImager (Molecular Dynamics, Sunnyvale, Calif.) and analyzed with ImageQuaNT version 4.1 (Molecular Dynamics). The program provides a volume measurement of each band based on the number and intensity of pixels. The values for iNOS were normalized by dividing the volume of the iNOS band by the volume of the HPRT band of the same sample and are expressed as relative units (iNOS/HPRT).
TABLE 2.
Oligonucleotide primers for mouse RT-PCR
| Primera | Sequence (reference)b | Product (bp) |
|---|---|---|
| IL-4 s | CTAGTTGTCATCCTGCTCTTCTTT | 380 |
| IL-4 a | CTTTAGGCTTTCCAGGAAGTCTTT | |
| IL-5 s | GTGAAAGAGACCTTGACACAGCTG | 290 |
| IL-5 a | CACACCAAGGAACTCTTGCAGGTA | |
| IL-10 s | TCAAACAAAGGACCAGCTGGACAA | 440 |
| IL-10 a | ATCAGATTTAGAGAGCTCTGTCTA | |
| IL-12p40 s | AGAGGTGGACTGGACTCCCGA | 588 |
| IL-12p40 a | CCTGATGAAGAAGCTGGTGCT | |
| IL-13 s | CTCCCTCTGACCCTTAAGGAG (34) | 311 |
| IL-13 a | GAAGGGGCCGTGGCGAAACAG | |
| IFN-γ s | TGTTACTGCCACGGCACAGTCATT | 362 |
| IFN-γ a | GTGGACCACTCGGATGAGCTCATT | |
| TNF-α s | GATCTCAAAGACAACCAACTAGTGc | 257 |
| TNF-α a | CTCCAGCTGGAAGACTCCTCCCAG | |
| TGF-β2 s | CACCACAAAGACAGGAACCTGG (42) | 329 |
| TGF-β2 a | GCGAAGGCAGCAATTATCCTGCAC | |
| iNOS s | CGGGCATTGCTCCCTTCCGAAAT | 428 |
| iNOS a | CTTCATGATAACGTTTCTGGCTCT | |
| KC s | GGATTCACCTCAAGAACATCCAGAG (2) | 454 |
| KC a | CACCCTTCTACTAGCACAGTGGTTG | |
| MIP-1α s | GAGAATTCGCGCCATATGGAGCTGACACCCCG | 225 |
| MIP-1α a | GAGATATCGGCAATCAGTTCCAGGTCAGTGAT | |
| MIP-2 s | CATCGAATTCGGCAGACTCCAGCCACACTTCAGCCT | 358 |
| MIP-2 a | GATCGGATCCGGCAGTTAGCCTTGCCTTTGTTCAGT | |
| MCP-1 s | TCCATGCAGGTCCCTGTCATGCTT (34) | 452 |
| MCP-1 a | CTAGTTCACTGTCACACTGGTC | |
| HPRT s | GTTGAATACAGGCCAGACTTTGTTG | 165 |
| HPRT a | GATTCAACTTGCGCTCATCTTAGGC |
s, sense; a, antisense.
Sequences are 5′ to 3′. All pairs except those referenced were designed by Frederick P. Heinzel.
Primer pair donated by Eric Pearlman, Case Western Reserve University.
Genotyping of KO mice.
To confirm the status of the IFN-γ KO and IL-12 KO mice, DNA was prepared from tail snips by using DNeasy spin columns (Qiagen, Valencia, Calif.) according to the manufacturer's instructions. For the iNOS KO mice, DNA was prepared from ≤10 mg of the snap-frozen spleens by using the spin columns. The DNA was eluted with 300 μl of elution buffer, and 2 μl was amplified with the primers in Table 3 by using PCRs with 50-μl reaction mixtures as described above. DNA was amplified for 30 cycles with an annealing temperature of 56°C.
TABLE 3.
Primers for genotyping IFN-γ−/−, IL-12p40−/−, and iNOS−/− mice
| Primer | Sequencea | Product (bp)
|
Reference | |
|---|---|---|---|---|
| WT | KO | |||
| IFN-γ 5′ | AGAAGTAAGTGGAAGGGCCCAGAAG | 225 | IMR126b | |
| IFN-γ 3′ | AGGGAAACTGGGAGAGGAGAAATAT | 225 | IMR127 | |
| Neomycin | TCAGCGCAGGGGCGCCCGGTTCTTT | 360 | IMR128 | |
| Neomycin | ATCGACAAGACCGGCTTCCATCCGA | 360 | IMR129 | |
| IL-12p40 5′ | AGTGAACCTCACCTGTGACACG | 680 | IMR457c | |
| IL-12p40 3′ | TCTTTGCACCAGCCATGAGC | 680 | IMR458 | |
| Neomycin | CTTGGGTGGAGAGGCTATTC | 280 | IMR013 | |
| Neomycin | AGGTGAGATGACAGGAGATC | 280 | IMR014 | |
| iNOS 5′ | ACATGCAGAATGAGTACCGG | 108 | IMR1216d | |
| iNOS 3′ | TCAACATCTCCTGGTGGAAC | 108 | IMR1217 | |
| Neomycin | AATATGCGAAGTGGACCTCG | 270 | IMR1218 | |
Sequences are 5′ to 3′.
TJL primer sequences for C57BL/6J-Ifngtm1Ts (stock no. 002287) are available at www.jax.org/resources/documents/imr/protocols/.
TJL primer sequences for C57BL/6J-Il12btm1Jm (stock no. 002693).
TJL primer sequences for C57BL/6J-Nos2tm1Lau (stock no. 002609).
Histologic evaluation.
Slides stained with hematoxylin and eosin were prepared by Histology Consultation Services (Everson, Wash.). Slides were coded and graded without knowledge of the experiment or treatment group, using the 0 to 5 scale as described recently (12). The slides were mixed randomly, graded, sorted according to score, and rechecked for consistency.
Statistics.
Within each experiment, all treatment groups of KO and WT mice were compared by analysis of variance and Fisher's protected least-significant-difference post hoc testing, using StatView 4.5 (Abacus Concepts, Berkeley, Calif.). The significance of the correlation between iNOS message and bacterial load was determined by correlation analysis with StatView 4.5.
RESULTS
Intranasal versus intragastric immunization.
Many immunization studies with mice have used intragastric administration of 1 to 4 mg of Helicobacter lysate. Other mucosal routes are also effective (20, 44). To compare intranasal to intragastric administration and to compare two different preparations of antigen, one produced from plate-cultured H. pylori (P) and the other produced from liquid-cultured H. pylori (L), two groups of mice were immunized intragastrically with 2 mg of sonicate P or L, and two groups of mice were immunized intranasally with 100 μg of the sonicates (Fig. 1). Two milligrams of sonicate was the usual dose used in this laboratory for intragastric administration (4), and the intranasal dose of sonicate (100 μg) was 10 times the amount of urease (10 μg) shown to be effective intranasally (20).
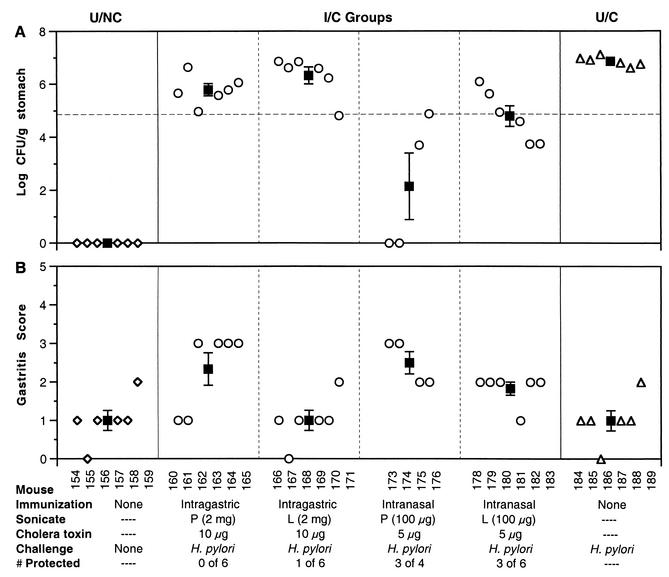
FIG. 1.
Comparison of protection and gastritis in mice immunized intragastrically or intranasally with P or L sonicate. Colonization (A) and gastritis (B) were determined 4 weeks after inoculation with 107 CFU of H. pylori SS1. Mice were immunized as described at the bottom. The horizontal dashed line in panel A is the protection level, i.e., a 2-log decrease in colonization from the mean of the U/C mice. Open symbols, individual mice; filled squares, group mean ± standard error of the mean. Mice immunized intranasally with the P sonicate showed the greatest protection (75%) and a relatively high level of gastritis.
Intranasal administration of the P sonicate produced the best protection, defined as a 2-log decrease in bacterial load compared to the U/C mice (Fig. 1A). Three of the four mice in that group had a greater-than-2-log-unit decrease in bacterial colonization (unfortunately two mice died of unrelated causes during the experiment). In two of the protected mice, no bacteria were detectable by culture. The group immunized intranasally with the L sonicate had three of six mice protected. Based on these results, intranasal immunization was more effective for both antigen preparations, and the P antigen produced better protection than the L antigen. All subsequent experiments used the intranasal route with the P antigen.
Interestingly, the gastritis scores of the mice immunized intragastrically with P antigen were similar to those of the intranasally immunized groups (Fig. 1B). In a kinetic study (12), increased gastric inflammation was associated with decreased bacterial load. This experiment demonstrated that the gastric inflammation may be increased in I/C mice (compared to U/NC and U/C mice) without a significant decrease in bacterial load at 4 weeks after challenge.
Cytokines expressed in the gastric mucosa.
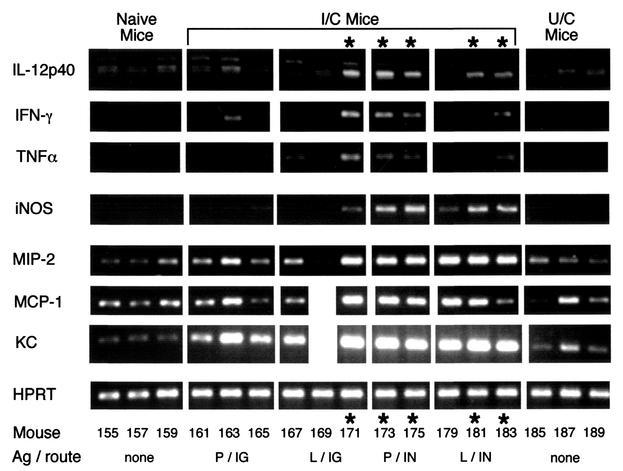
Since some of the immunized mice in the antigen-route experiment were protected while others were not, these samples were used to determine which cytokines expressed in the gastric mucosa were associated with protection. RNA was isolated from the frozen stomachs of the mice shown in Fig. 1 and reverse transcribed as described in Materials and Methods. PCRs were performed with the primer pairs shown in Table 2. Initially, half the samples (even-numbered mice) were analyzed for IL-4, IL-5, IFN-γ, iNOS, and HPRT (data not shown). Message for IFN-γ and iNOS was increased in the protected mice. The remaining samples were then analyzed for the entire panel of cytokines (Table 2), and results for the products that varied with treatment group and protection are shown in Fig. 2. The protected I/C mice had greater amounts of message for IL-12p40, IFN-γ, TNF-α, and iNOS than naive (U/NC) mice, unprotected I/C mice, and U/C mice. The HPRT (housekeeping gene) products were similar for all mice, indicating that the amount of cDNA amplified was approximately equivalent for each mouse. There was greater expression of the chemokines, MIP-2, MCP-1, and KC, in the immunized groups than in U/NC and U/C mice. However, some of the unprotected I/C mice had equally high expression of chemokines as the protected mice. Thus, expression of chemokines appeared to be associated with immunization rather than protection. Products for IL-4 and IL-13 were barely detectable and did not vary with the different treatments (data not shown). Message for TGF-β was abundant in all mice; products for IL-5, IL-10, and MIP-1α were not associated with treatment or protection (data not shown). In retrospect, it is not surprising that all samples had high levels of message for TGF-β, since normal gastric epithelial cells express TGF-β (24).
FIG. 2.
Increased levels of IL-12p40, IFN-γ, TNF-α, and iNOS are associated with protection. Gastric mRNA from the mice shown in Fig. 1 was analyzed by semiquantitative RT-PCR, and results for representative (odd-numbered) mice are shown. Asterisks indicate the lanes from protected mice. RNA was isolated from frozen stomachs by using RNA STAT-60. Three micrograms of total RNA was reverse transcribed with oligo(dT)12-18 and RNase H− reverse transcriptase. Ten percent of the RT reaction mixture was amplified with each pair of primers for 35 cycles, except HPRT, which was amplified for 25 cycles as a control to demonstrate that the amount of starting cDNA was approximately equivalent for each mouse. IG, intragastric; IN, intranasal.
Association of iNOS message and clearance of bacteria.
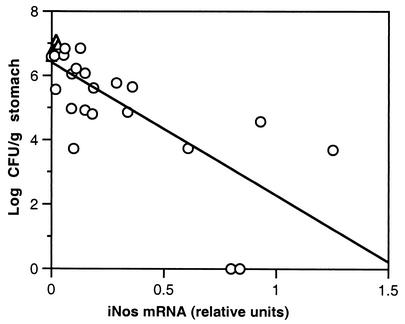
The fact that iNOS was expressed at a higher level in the protected I/C mice suggested that nitric oxide (NO) may play a role in the clearance of bacteria. NO has an antimicrobial effect on many pathogens (10) and converted H. pylori from the spiral replicating form to the coccoid nonreplicating form (5). The relationship of iNOS expression to bacterial load was further analyzed in the challenged mice shown in Fig. 1 by densitometry. The values for the iNOS bands were normalized by using the value of the HPRT band of the same sample and are shown as relative units in Fig. 3. Although there was considerable variability, iNOS expression was inversely related to bacterial load (P < 0.0001).
FIG. 3.
Correlation of bacterial load and gastric mRNA for iNOS. Relative units of iNOS mRNA/HPRT mRNA were determined by RT-PCR for the 28 challenged mice shown in Fig. 1 as described in Materials and Methods. ▵, U/C mice; ○, I/C mice. There was an inverse correlation between bacterial load and iNOS mRNA (r = 0.742).
Studies of protection in gene-targeted mice.
Analysis of gene expression by RT-PCR is only semiquantitative, and the association of cytokine expression with protection does not prove a cause-and-effect relationship. To determine the biologic effects of IFN-γ, IL-12p40, and iNOS, mice lacking these products were immunized according to the optimal protocol from the antigen-route experiment, i.e., intranasally with four weekly doses of 5 μg of CT mixed with 100 μg of sonicate P. A time point of 4 weeks postchallenge was chosen because a kinetic study using this immunization protocol had shown a greater decrease in colonization at 4 weeks than at 2 weeks postchallenge (12). The effect of the adjuvant, CT, on colonization by H. pylori was studied by immunizing some of the WT, IFN-γ KO, and IL-12 KO mice with a mixture of CT and ovalbumin.
Protection in iNOS-deficient mice.
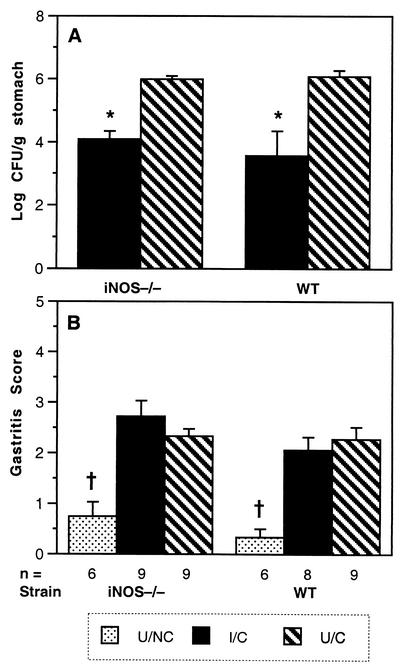
Despite the association of iNOS message with a decrease in bacterial load, mice with a targeted disruption of the iNOS gene which prevents the production of active enzyme (22) had reductions in bacterial load similar to those of WT mice after immunization (Fig. 4A). The gastritis scores of the I/C and U/C iNOS KO and WT mice were significantly higher than those of the U/NC mice (P < 0.0001) but were not different from one another (Fig. 4B). To be certain that the mice had been identified correctly upon arrival, their genetic status was confirmed by genotyping as described in Materials and Methods. These results indicate that iNOS does not play a significant role in reducing colonization after immunization.
FIG. 4.
Immunized mice lacking iNOS show a decrease in bacterial load similar to that in WT mice. (A) Colonization, shown as geometric mean ± standard error of the mean, was measured 4 weeks after challenge with 107 CFU of H. pylori SS1. Mice were immunized intranasally with 100 μg of sonicate (P) and 5 μg of CT as described in Materials and Methods. In both strains, I/C mice had a significant decrease in bacterial load. *, P ≤ 0.0015. U/NC mice were culture negative in all experiments (not shown). (B) Gastritis, shown as mean ± standard error of the mean, was significantly lower in both U/NC groups than in all challenged groups. †, P < 0.0001.
Protection in IFN-γ-deficient mice.
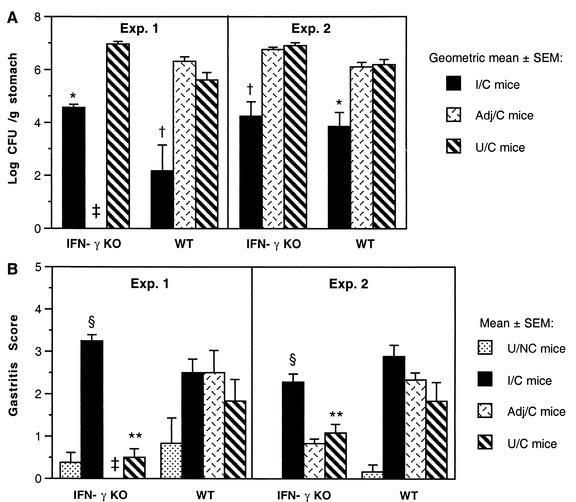
Reports on the requirement for IFN-γ in protective immunity are inconsistent. In an H. felis study, mice deficient in IFN-γ receptor were not protected by prophylactic immunization [F. J. Radcliff, A. J. Ramsay, and A. Lee, abstract from the 9th International Congress of Mucosal Immunology 1997, Immunol. Cell Biol., 75(Suppl. 1):A90, 1997]. In a more recent study, eight of nine orally immunized IFN-γ KO mice were protected after challenge with H. pylori (35). Since IFN-γ expression was elevated in protected mice (Fig. 2), we also immunized and challenged IFN-γ KO mice. In two experiments, the I/C IFN-γ KO mice had a >2-log reduction in bacterial colonization compared to U/C IFN-γ KO mice (Fig. 5A). The mean colonization of the U/C and Adj/C IFN-γ KO mice was higher than the colonization in the corresponding WT groups, although the difference was not statistically different. Gastritis scores were significantly lower in the U/C and Adj/C IFN-γ KO mice than in challenged WT mice (Fig. 5B). In contrast, the gastritis in the I/C IFN-γ KO mice was similar to the gastritis in I/C WT mice and significantly higher than that in other IFN-γ KO groups (P < 0.001). The disruption of the gene for IFN-γ was confirmed by genotyping the breeders and each of the IFN-γ KO mice in the second experiment. These results indicate that IFN-γ is not required for protection against H. pylori or for the development of gastric inflammation in immunized mice.
FIG. 5.
Mice lacking IFN-γ show a greater-than-2-log-unit reduction in bacterial load and develop gastritis after immunization and challenge. Mice were immunized intranasally with 100 μg of sonicate and 5 μg of CT as described in Materials and Methods and Table 1. Adj/C mice received 100 μg of ovalbumin and 5 μg of CT. Colonization (A) and gastritis (B) were determined 4 weeks after inoculation with H. pylori SS1. In both experiments, the I/C mice had decreased colonization compared to the Adj/C and U/C mice (*, P < 0.015; †, P ≤ 0.0001). Gastritis in the I/C IFN-γ KO mice was higher than that in the U/NC and U/C IFN-γ KO groups (§, P < 0.001). The U/C IFN-γ KO mice had less gastritis than challenged WT mice (**, P < 0.05). ‡, not done.
Importance of IL-12p40 in protective immunity.
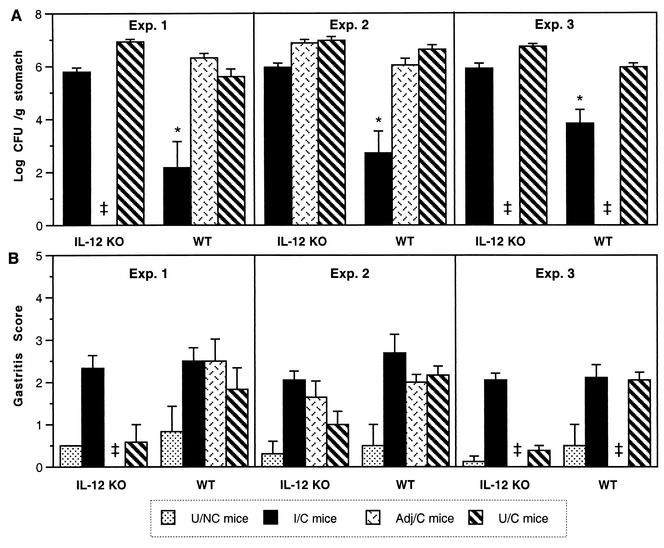
In three independent immunization-challenge experiments with IL-12p40 KO mice, the I/C mice were not protected (Fig. 6A). In each experiment there was a small (<1 log) decrease in bacterial load compared to the U/C or Adj/C IL-12 KO mice, but this decrease was not statistically significant in experiments 2 and 3, and not one individual I/C IL-12 KO mouse had a ≥2-log decrease in colonization (the definition of protection used in this study). WT mice immunized at the same time with the same antigen preparation in each experiment were protected (P < 0.0001). Thus, IL-12p40 appears to play an important role in the development of protection against H. pylori.
FIG. 6.
I/C mice lacking IL-12p40 show less than a 10-fold decrease in bacterial load but develop gastritis similar to that in challenged WT mice. Mice were immunized intranasally with 100 μg of sonicate and 5 μg of CT as described in Materials and Methods and Table 1. Adj/C mice received 100 μg of ovalbumin and 5 μg of CT. Colonization (A), shown as geometric mean ± standard error of the mean, and gastritis (B), shown as mean ± standard error of the mean, were determined 4 weeks after inoculation with H. pylori SS1. In three experiments, the WT I/C mice had decreased colonization compared to the Adj/C and U/C mice (*, P ≤ 0.0001). ‡, not done.
The I/C IL-12 KO mice had gastritis scores similar to those in the three groups of challenged WT mice (Fig. 6B). In two of the three experiments, the U/C IL-12 KO mice had significantly less gastric inflammation than the U/C WT mice. The development of gastric inflammation in I/C IL-12 KO mice is not dependent on IL-12p40.
Histologic appearance of gastritis in IFN-γ KO, IL-12 KO, and WT mice.
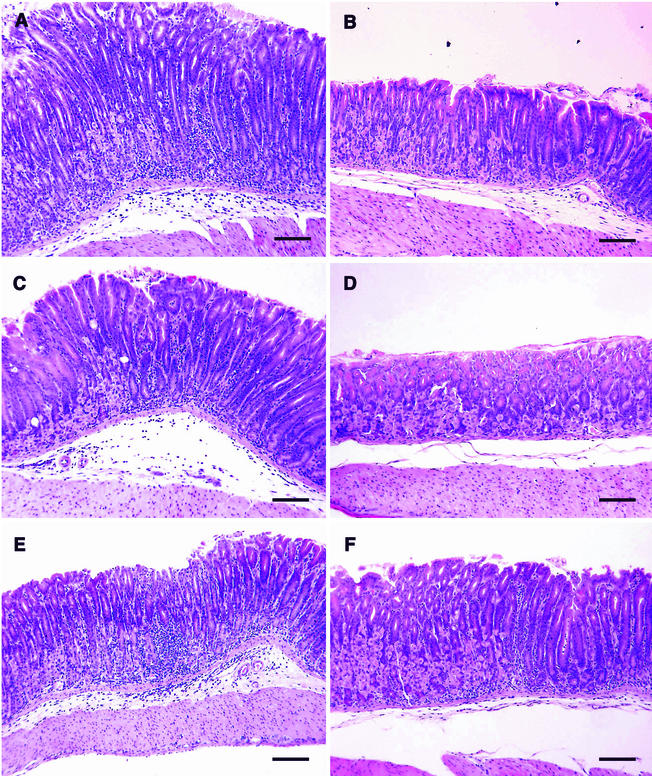
Representative histologic sections from mice in experiment 1 (Table 1) are shown in Fig. 7. The magnification is the same in each photomicrograph, and differences in the thickness of the sections are due to inflammatory infiltration in the mucosa and submucosa and epithelial cell hyperplasia. All photomicrographs were taken at the junction of the body and antrum, where the worst lesions are usually observed in C57BL/6 mice with H. pylori-induced gastritis (12). The infiltrate in I/C mice (IFN-γ KO [Fig. 7A], IL-12 KO [Fig. 7C], and WT [Fig. 7E]) and U/C WT mice (Fig. 7F) consisted of neutrophils, macrophages, plasma cells, eosinophils, and lymphocytes. In areas of greatest infiltration, the parietal and chief cells were absent and glands were displaced and obscured by the infiltrate. The height of the glands, especially in Fig. 7A, was increased by hyperplasia of epithelial cells. Although not discernible at this magnification, many of the infiltrating cells in the edematous submucosa in Fig. 7A, C, and E were plasma cells. There was slight or no inflammation in the sections from U/C IFN-γ KO (Fig. 7B) and U/C IL-12 KO (Fig. 7D) mice, respectively.
FIG. 7.
Representative photomicrographs of stomachs from the IFN-γ KO, IL-12 KO, and WT experiment (Table 1, experiment 1). (A and B) IFN-γ KO mice; (C and D) IL-12 KO mice on the C57BL/6J background; (E and F) WT C57BL/6 mice. Stomachs were collected 4 weeks after challenge with H. pylori SS1. (A, C, and E) Intranasally immunized; (B, D, and F) unimmunized. Group means are shown as experiment 1 in Fig. 5 and 6. Gastritis scores are as follows: 3.5 (A), 0.5 (B), 2.5 (C), 0 (D), 3.0 (E), and 2.0 (F). Bars, 100 μm. Hematoxylin and eosin staining was used.
Adjuvant alone has no effect on bacterial clearance.
The adjuvant effects of CT when administered with ovalbumin did not result in a decrease in bacterial colonization compared to that in the U/C mice in three experiments with WT mice (Fig. 5A, experiments 1 and 2 and Fig. 6A, experiment 2 [note that the WT mice of experiment 1 are shown in both Fig. 5 and 6 for comparison with the respective KO mice]). Also, colonization was not decreased by immunization with CT-ovalbumin in IFN-γ KO mice (Fig. 5A, experiment 2) or in IL-12 KO mice (Fig. 6.A, experiment 2). Gastritis scores in Adj/C mice were not significantly different from those in U/C mice (Fig. 5B and 6B). Under the conditions used in these experiments (intranasal prophylactic immunization and a 4-week time point), CT itself did not have an effect on bacterial load or gastritis resulting from inoculation with H. pylori.
DISCUSSION
The most significant finding in this study is that IL-12p40 plays a role in the reduction of bacterial load in I/C mice and appears to do so in a manner that is not strictly dependent on IFN-γ. IL-12 is a heterodimer of two disulfide-linked subunits, p35 and p40, made mainly by phagocytic cells and to a lesser extent by B cells (40, 43). It plays a primary role in innate immunity as well as adaptive immunity. It induces NK and T cells to produce IFN-γ and proliferate. The effects of IL-12 are not due to IFN-γ induction alone. Treatment with IFN-γ in vivo does not mimic treatment with IL-12 (40). IL-12 can also stimulate the production of TNF-α, granulocyte-macrophage colony-stimulating factor, and IL-8 (43). Based on the differences in protection in the absence of IFN-γ and IL-12, it seems likely that IL-12, as a more upstream cytokine, can induce the formation of other mediators which can reduce bacterial load in the absence of IFN-γ. IL-12 also enhances humoral immunity (27), but since antibodies are not required for protection from H. pylori, lack of protection in the absence of IL-12 is not likely to be due to impairment of humoral immunity.
The two subunits of IL-12 are encoded by separate genes on different chromosomes. The p35 subunit is expressed by many cell types constitutively, but the p40 subunit is made only in macrophages, B cells, and dendritic cells. Only cells which produce the p40 subunit produce the biologically active heterodimer IL-12p70. Recently, the p40 portion of IL-12 has been reported to interact with a novel p19 protein to form IL-23 (32). Similar to p35, the p19 polypeptide is not bioactive until it combines with p40. IL-23 is secreted by activated dendritic cells. IL-23 has some effects similar to those of IL-12, such as the stimulation of IFN-γ production, but also uniquely induces proliferation of mouse memory T cells. Since the IL-12 primers used in our RT-PCR were specific for p40 and the IL-12 KO mice were deficient in p40, we have not distinguished IL-12 from IL-23; either or both may play a major role in reduction of bacterial load.
The two experiments with IFN-γ KO mice presented here confirm the findings of Sawai et al. (35) and are in disagreement with the report by Radcliff et al. [Immunol. Cell Biol., 75(Suppl. 1):A90, 1997]. Sawai et al. showed that a greater number of H. pylori strains were able to colonize IFN-γ KO mice than WT mice and that the IFN-γ KO mice had higher levels of colonization (35). Kamradt et al. also reported greater colonization in IFN-γ KO mice than in WT mice on both C57BL/6 and BALB/c backgrounds (18). In agreement with these studies, the mean colonization in U/C IFN-γ KO mice was higher than that in WT mice in both experiments presented here. Also, similar to the results of Sawai et al. (35), we found that gastric inflammation was reduced in U/C IFN-γ KO mice compared to WT mice. These observations suggest that endogenous IFN-γ plays a role in controlling the level of colonization and inflammation in unimmunized mice. This was not surprising, since treatment with anti-IFN-γ neutralizing antibodies decreased the inflammation elicited by H. felis in C57BL/6 mice (28). Also, IFN-γ is the predominant cytokine made by T cells isolated from the inflamed mucosa of H. pylori-infected patients (3, 39).
Based on the increased colonization and lack of inflammation in mice without endogenous IFN-γ, one might predict that mice lacking IFN-γ would not be protected from H. pylori infection by immunization. However, orally immunized IFN-γ KO mice were protected from H. pylori (35). The two experiments presented here confirm that the immunization-induced reduction in bacterial load does not require IFN-γ and add the observation that the development of gastritis in I/C mice does not require IFN-γ (Fig. 5B). The I/C IFN-γ KO mice had inflammation similar to that of the I/C WT mice and had significantly greater inflammation than the U/C IFN-γ mice. An intriguing question is what is causing the IFN-γ-independent inflammation in immunized mice. A few of the possible mediators include IL-1β, TNF-α, and IL-6.
The increased levels of iNOS message in the stomachs of protected mice suggested that NO production might be a mechanism of clearing H. pylori. NO is produced by macrophages and is highly diffusible, although its half-life is very short. It has been shown to be important in killing intracellular pathogens such as Leishmania and Mycobacterium tuberculosis (10). However, decreased colonization in the I/C iNOS KO mice clearly showed that the immunization-induced reduction in bacterial load does not require iNOS. The effects of increased NO production by iNOS vary with the disease process and range from beneficial to the host to detrimental (30). In a rat model of gastric injury induced by ethanol, NO had a gastroprotective effect that was both direct and indirect via enhanced production of prostaglandin E2 (21). Also, iNOS mediated early epithelial repair after experimental injury to the colonic mucosa (13). In a bacterial colitis model, infected mice had increased levels of iNOS activity until they cleared the infection, but iNOS−/− mice controlled and cleared the infection similarly to the WT mice, indicating that iNOS was not essential to host defense (37). It is possible that the high levels of iNOS in our I/C protected mice were a response to the gastric inflammation induced by H. pylori.
Intranasal immunization in this study produced better protection than intragastric immunization. This may be due at least in part to the difference in dosage. A 2-mg dose may not be the optimal intragastric dose for this antigen preparation. Antigen dose, timing, and number of administrations have each been shown to affect protection against H. pylori in mice (41). The intranasal route may work better than intragastric immunization by permitting greater contact with lymphoid tissue which is abundant beneath the nasal and pharyngeal epithelium (44). The normal mouse stomach has very little mucosa-associated lymphoid tissue, and the intragastric immune response may be stimulated by antigen that passes through the stomach and comes into contact with the intestinal mucosa-associated lymphoid tissue. Although sodium bicarbonate is used to neutralize stomach acid, some degradation of the antigen is likely. Regardless of whether intranasal immunization is inherently better, it has the practical advantage of allowing the use of smaller doses of antigen and CT. Thus, the same preparation of antigen can be tested and used for multiple experiments. Because the sonicate is a crude preparation, using a single preparation helps remove concern that differences between experiments may be due to differences in the antigen preparation. It is possible that the plate-cultured antigen elicited better protection because it contained more proteins, especially urease, released by the bacteria. The first step in the preparation of the liquid-cultured antigen was to pellet the bacteria from culture medium, leaving the released proteins in the discarded supernatant.
Finally, a protective effect of the CT adjuvant was not observed in our WT, IFN-γ KO, or IL-12 KO mice at 4 weeks after challenge. Recently, a significant effect of CT was reported in a therapeutic immunization study (17). H. pylori eradication in mice treated orally with 5 μg of CT was 47%, compared to 62% in mice immunized with sonicate and CT 6 weeks after CT administration. The different results may be due to timing (we looked at only a 4-week time point), but most likely the immunization protocol is important, as Ikewaki et al. suggested in their discussion (17). When mice are immunized therapeutically with CT alone, there is a population of H. pylori in the stomach releasing H. pylori antigens. Therefore, CT may be taken up simultaneously with H. pylori antigens to produce an immune response similar to but less effective than the response to immunization with premixed H. pylori antigens and CT. In our prophylactic immunization protocol, H. pylori was not present when the CT-ovalbumin mixture was given.
In summary, the experiments presented here have shown that protected mice have greater gastric expression of message for IL-12p40, IFN-γ, TNF-α, and iNOS than immunized mice which were not protected, but iNOS and IFN-γ are not required for protection. Mice lacking IL-12p40 were not protected by immunization. The biologic effects of IL-12 and IL-23 should be evaluated further.
Acknowledgments
This work was supported in part by Public Health Service grants CA-73515 (to C.A.G.), DK-46461 (to S.J.C. and J.G.N.), AI-40701 (to J.G.N.), and AI-36359 (to J.G.N. and S.J.C.). F.P.H. is supported by grants AI45602 and AI35979 and by the VA Medical Research Service.
We thank Steven Emancipator, Raymond Redline, and Abram Stavitsky for their advice and many helpful discussions. We appreciate the assistance of Nancy Edgehouse and LeRoy Brown in preparing the histologic sections.
Editor: D. L. Burns
REFERENCES
- 1.Agnihotri, N., D. Bhasin, H. Vohra, P. Ray, K. Singh, and N. Ganguly. 1998. Characterization of lymphocytic subsets and cytokine production in gastric biopsy samples from Helicobacter pylori patients. Scand. J. Gastroenterol. 33:704-709. [DOI] [PubMed] [Google Scholar]
- 2.Ajuebor, M. N., A. M. Das, L. Virag, R. J. Flower, C. Szabo, and M. Perretti. 1999. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J. Immunol. 162:1685-1691. [PubMed] [Google Scholar]
- 3.Bamford, K. B., X. Fan, S. E. Crowe, J. F. Leary, W. K. Gourley, G. K. Luthra, E. G. Brooks, D. Y. Graham, V. E. Reyes, and P. B. Ernst. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114:482-492. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard, T. G., S. J. Czinn, R. W. Redline, N. Sigmund, G. Harriman, and J. G. Nedrud. 1999. Antibody-independent protective mucosal immunity to gastric Helicobacter infection in mice. Cell. Immunol. 191:74-80. [DOI] [PubMed] [Google Scholar]
- 5.Cole, S. P., V. F. Kharitonov, and D. G. Guiney. 1999. Effect of nitric oxide on Helicobacter pylori morphology. J. Infect. Dis. 180:1713-1717. [DOI] [PubMed] [Google Scholar]
- 6.Crabtree, J. E., T. M. Shallcross, R. V. Heatley, and J. I. Wyatt. 1991. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut 32:1473-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crabtree, J. E., J. I. Wyatt, L. K. Trejdosiewicz, P. Peichl, P. H. Nichols, N. Ramsay, J. N. Primrose, and I. J. Lindley. 1994. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J. Clin. Pathol. 47:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Elios, M. M., M. Manghetti, M. De Carli, F. Costa, C. T. Baldari, D. Burroni, J. L. Telford, S. Romagnani, and G. Del Prete. 1997. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962-967. [PubMed] [Google Scholar]
- 9.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Myers, J. Nedrud, R. Weltzin, C. K. Lee, H. Kleanathous, and T. P. Monath. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 188:2277-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang, F. C. 1997. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Invest. 99:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox, J. G., P. Beck, C. A. Dangler, M. T. Whary, T. C. Wang, H. N. Shi, and C. Nagler-Anderson. 2000. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 6:536-542. [DOI] [PubMed] [Google Scholar]
- 12.Garhart, C. A., R. W. Redline, J. G. Nedrud, and S. J. Czinn. 2002. Clearance of Helicobacter pylori infection and resolution of postimmunization gastritis in a kinetic study of prophylactically immunized mice. Infect. Immun. 70:3529-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gookin, J. L., J. M. Rhoads, and R. A. Argenzio. 2002. Inducible nitric oxide synthase mediates early epithelial repair of porcine ileum. Am. J. Physiol. Gastrointest. Liver Physiol. 283:G157-G168. [DOI] [PubMed] [Google Scholar]
- 14.Goto, T., A. Nishizono, T. Fujioka, J. Ikewaki, K. Mifune, and M. Nasu. 1999. Local secretory immunoglobulin A and postimmunization gastritis correlate with protection against Helicobacter pylori infection after oral vaccination of mice. Infect. Immun. 67:2531-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottwein, J. M., T. G. Blanchard, O. S. Targoni, J. C. Eisenberg, B. M. Zagorski, R. W. Redline, J. G. Nedrud, M. Tary-Lehmann, P. V. Lehmann, and S. J. Czinn. 2001. Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete Freund's adjuvant by systemic immunization. J. Infect. Dis. 184:308-314. [DOI] [PubMed] [Google Scholar]
- 16.Ibraghimov, A., and J. Pappo. 2000. The immune response against Helicobacter pylori—a direct linkage to the development of gastroduodenal disease. Microbes Infect. 2:1073-1077. [DOI] [PubMed] [Google Scholar]
- 17.Ikewaki, J., A. Nishizono, T. Goto, T. Fujioka, and K. Mifune. 2000. Therapeutic oral vaccination induces mucosal immune response sufficient to eliminate long-term Helicobacter pylori infection. Microbiol. Immunol. 44:29-39. [DOI] [PubMed] [Google Scholar]
- 18.Kamradt, A. E., M. Greiner, P. Ghiara, and S. H. Kaufmann. 2000. Helicobacter pylori infection in wild-type and cytokine-deficient C57BL/6 and BALB/c mouse mutants. Microbes Infect. 2:593-597. [DOI] [PubMed] [Google Scholar]
- 19.Karttunen, R. A., T. J. Karttunen, M. M. Yousfi, H. El-Zimaity, D. Y. Graham, and F. El-Zaatari. 1997. Expression of mRNA for interferon-gamma, interleukin-10, and interleukin-12 (p40) in normal gastric mucosa and in mucosa infected with Helicobacter pylori. Scand. J. Gastroenterol. 32:22-27. [DOI] [PubMed] [Google Scholar]
- 20.Kleanthous, H., G. A. Myers, K. M. Georgakopoulos, T. J. Tibbitts, J. W. Ingrassia, H. L. Gray, R. Ding, Z. Z. Zhang, W. Lei, R. Nichols, C. K. Lee, T. H. Ermak, and T. P. Monath. 1998. Rectal and intranasal immunizations with recombinant urease induce distinct local and serum immune responses in mice and protect against Helicobacter pylori infection. Infect. Immun. 66:2879-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konturek, P. C., T. Brzozowski, Z. Sliwowski, R. Pajdo, J. Stachura, E. G. Hahn, and S. J. Konturek. 1998. Involvement of nitric oxide and prostaglandins in gastroprotection induced by bacterial lipopolysaccharide. Scand. J. Gastroenterol. 33:691-700. [DOI] [PubMed] [Google Scholar]
- 22.Laubach, V. E., E. G. Shesely, O. Smithies, and P. A. Sherman. 1995. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc. Natl. Acad. Sci. USA 92:10688-10692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 24.Lindholm, C., M. Quiding-Jarbrink, H. Lonroth, A. Hamlet, and A. Svennerholm. 1998. Local cytokine response in Helicobacter pylori-infected subjects. Infect. Immun. 66:5964-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohoff, M., M. Rollinghoff, and F. Sommer. 2000. Helicobacter pylori gastritis: a Th1 mediated disease? J. Biotechnol. 83:33-36. [DOI] [PubMed] [Google Scholar]
- 26.Lucas, B., D. Bumann, A. Walduck, J. Koesling, L. Develioglu, T. F. Meyer, and T. Aebischer. 2001. Adoptive transfer of CD4+ T cells specific for subunit A of Helicobacter pylori urease reduces H. pylori stomach colonization in mice in the absence of interleukin-4 (IL-4)/IL-13 receptor signaling. Infect. Immun. 69:1714-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metzger, D. W., R. M. McNutt, J. T. Collins, J. M. Buchanan, V. H. Van Cleave, and W. A. Dunnick. 1997. Interleukin-12 acts as an adjuvant for humoral immunity through interferon-gamma-dependent and -independent mechanisms. Eur. J. Immunol. 27:1958-1965. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi, M., S. Czinn, R. Redline, and J. Nedrud. 1996. Helicobacter-specific cell-mediated immune responses display a predominant TH1 phenotype and promote a DTH response in the stomachs of mice. J. Immunol. 156:4729-4738. [PubMed] [Google Scholar]
- 29.Mohammadi, M., J. Nedrud, R. Redline, N. Lycke, and S. Czinn. 1997. Murine CD4 T cell responses to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology 113:1848-1857. [DOI] [PubMed] [Google Scholar]
- 30.Nathan, C. 1997. Inducible nitric oxide synthase: what difference does it make? J. Clin. Invest. 100:2417-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noach, L. A., N. B. Bosma, J. Jansen, F. J. Hoek, S. J. van Deventer, and G. N. Tytgat. 1994. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand. J. Gastroenterol. 29:425-429. [DOI] [PubMed] [Google Scholar]
- 32.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. de Waal-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715-725. [DOI] [PubMed] [Google Scholar]
- 33.Pappo, J., D. Torrey, L. Castriotta, A. Savinainen, Z. Kabok, and A. Ibraghimov. 1999. Helicobacter pylori infection in immunized mice lacking major histocompatibility complex class I and class II functions. Infect. Immun. 67:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearlman, E., J. Lass, D. Bardenstein, E. Diaconu, F. Hazlett, J. Albright, A. Higgins, and J. Kazura. 1997. IL-12 exacerbates helminth-mediated corneal pathology by augmenting inflammatory cell recruitment and chemokine expression. J. Immunol. 158:827-833. [PubMed] [Google Scholar]
- 35.Sawai, N., M. Kita, T. Kodama, T. Tanahashi, Y. Yamaoka, Y.-I. Tagawa, Y. Iwakura, and J. Imanishi. 1999. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect. Immun. 67:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimoyama, T., S. M. Everett, M. F. Dixon, A. T. Axon, and J. E. Crabtree. 1998. Chemokine mRNA expression in gastric mucosa is associated with Helicobacter pylori cagA positivity and severity of gastritis. J. Clin. Pathol. 51:765-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmons, C. P., N. S. Goncalves, M. Ghaem-Maghami, M. Bajaj-Elliott, S. Clare, B. Neves, G. Frankel, G. Dougan, and T. T. MacDonald. 2002. Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-γ. J. Immunol. 168:1804-1812. [DOI] [PubMed] [Google Scholar]
- 38.Smythies, L. E., K. B. Waites, J. R. Lindsey, P. R. Harris, P. Ghiara, and P. D. Smith. 2000. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J. Immunol. 165:1022-1029. [DOI] [PubMed] [Google Scholar]
- 39.Sommer, F., G. Faller, P. Konturek, T. Kirchner, E. G. Hahn, J. Zeus, M. Rollinghoff, and M. Lohoff. 1998. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect. Immun. 66:5543-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stern, A. S., J. Magram, and D. H. Presky. 1996. Interleukin-12 an integral cytokine in the immune response. Life Sci. 58:639-654. [DOI] [PubMed] [Google Scholar]
- 41.Sutton, P., J. Wilson, and A. Lee. 2000. Further development of the Helicobacter pylori mouse vaccination model. Vaccine 18:2677-2685. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi, M., M. M. Kosiewicz, P. Alard, and J. W. Streilein. 1997. On the mechanisms by which transforming growth factor-beta 2 alters antigen-presenting abilities of macrophages on T cell activation. Eur. J. Immunol. 27:1648-1656. [DOI] [PubMed] [Google Scholar]
- 43.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251-276. [DOI] [PubMed] [Google Scholar]
- 44.Weltzin, R., H. Kleanthous, F. Guirdkhoo, T. P. Monath, and C. K. Lee. 1997. Novel intranasal immunization techniques for antibody induction and protection of mice against gastric Helicobacter felis infection. Vaccine 15:370-376. [DOI] [PubMed] [Google Scholar]
- 45.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, and J. Imanishi. 1996. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology 110:1744-1752. [DOI] [PubMed] [Google Scholar]
- 46.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, K. Kashima, and J. Imanishi. 1997. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut 41:442-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, T. Tanahashi, K. Kashima, and J. Imanishi. 1998. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut 42:609-617. [DOI] [PMC free article] [PubMed] [Google Scholar]