Abstract
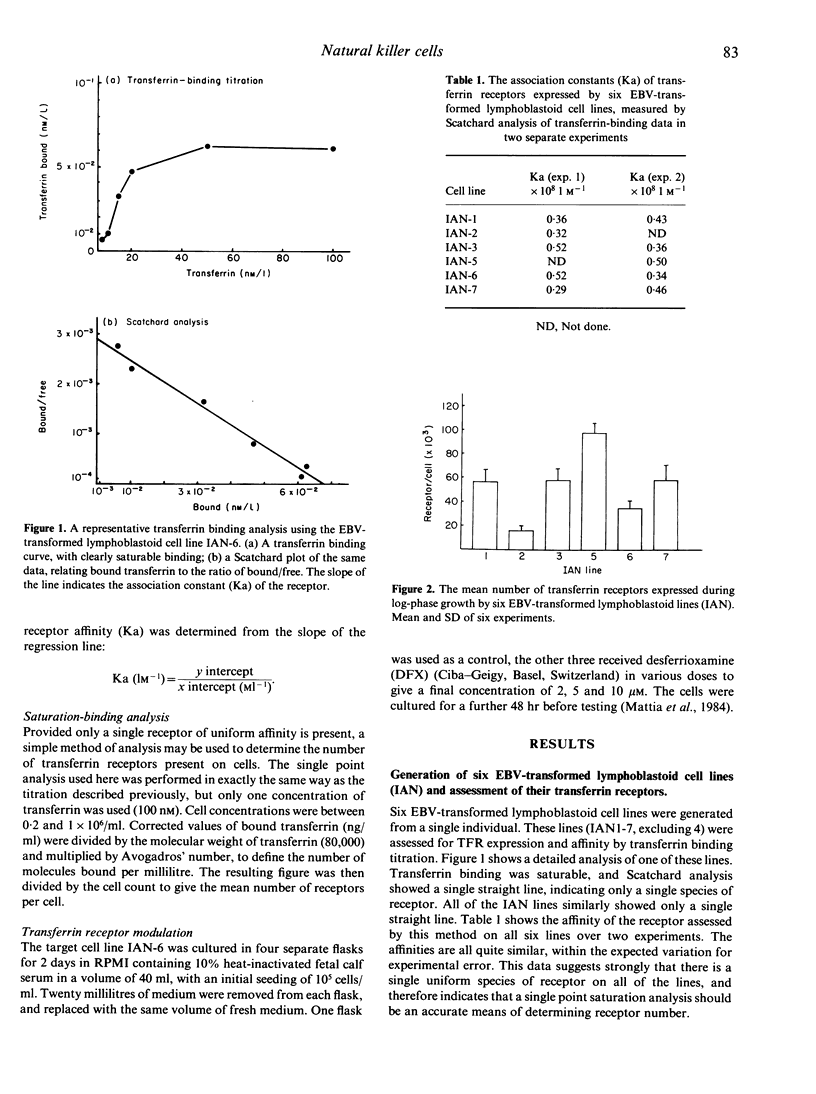
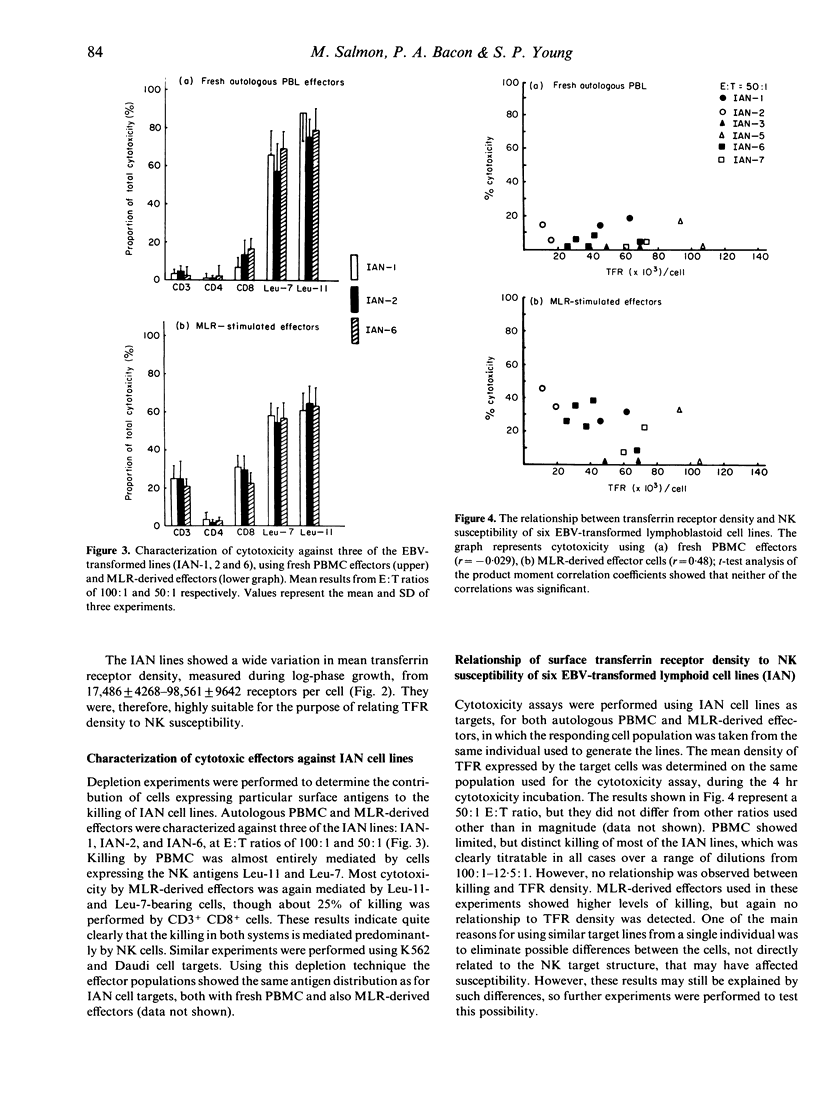
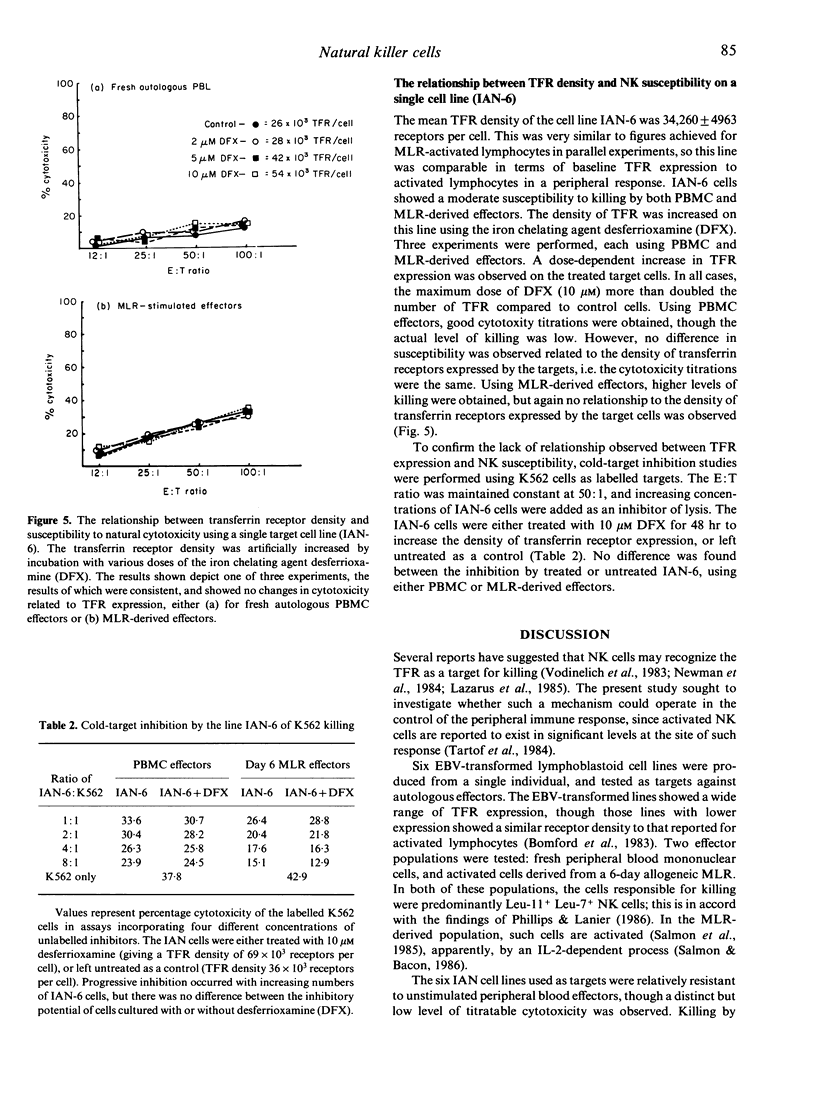
Several reports suggest that natural killer (NK) cells recognize the transferrin receptor (TFR) as a target for killing, and that natural cytotoxicity may be involved in the control of stem cell proliferation in bone-marrow. This study tested whether NK-cell recognition of the TFR on activated lymphocytes plays a role in the control of peripheral immune responses. Six lymphoid lines were created from a single individual, and used as targets for cytotoxicity assays, using either peripheral blood mononuclear cells, or mixed lymphocyte reaction (MLR)-derived effectors. The cells responsible for killing were predominantly Leu-11+Leu-7+ NK cells, though CD3+ cells accounted for about 25% of cytotoxicity from MLR. No correlation was observed between TFR density and NK susceptibility when using all six cell lines. Specifically increasing the density of TFR on a single cell line failed to increase susceptibility to NK, suggesting that the TFR does not act as a major target for natural cytotoxicity directed at lymphoid cells. Furthermore, the relatively low levels of killing observed indicate that activated NK populations that accumulate at sites of immune response are unlikely to play a direct immunoregulatory role.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D., Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. 1980 Mar 1;102(2):255–270. doi: 10.1016/0003-2697(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Biron C. A., Welsh R. M. Activation and role of natural killer cells in virus infections. Med Microbiol Immunol. 1982;170(3):155–172. doi: 10.1007/BF02298196. [DOI] [PubMed] [Google Scholar]
- Bomford A., Young S. P., Nouri-Aria K., Williams R. Uptake and release of transferrin and iron by mitogen-stimulated human lymphocytes. Br J Haematol. 1983 Sep;55(1):93–101. doi: 10.1111/j.1365-2141.1983.tb01227.x. [DOI] [PubMed] [Google Scholar]
- Bridges K. R., Smith B. R. Discordance between transferrin receptor expression and susceptibility to lysis by natural killer cells. J Clin Invest. 1985 Sep;76(3):913–918. doi: 10.1172/JCI112089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield M. J., Cerny J. Cell interactions in leukemia-associated immunosuppression: suppression of thymus-independent antibody responses by leukemia spleen cells (Moloney) in vitro is mediated by normal T cells. J Immunol. 1980 Jan;124(1):255–260. [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Frost P., Smith J., Frost H. The radiolabeling of lymphocytes and tumor cells with 111indium. Proc Soc Exp Biol Med. 1978 Jan;157(1):61–65. doi: 10.3181/00379727-157-39991. [DOI] [PubMed] [Google Scholar]
- Holmberg L. A., Miller B. A., Ault K. A. The effect of natural killer cells on the development of syngeneic hematopoietic progenitors. J Immunol. 1984 Dec;133(6):2933–2939. [PubMed] [Google Scholar]
- Lazarus A. H., Baines M. G. Studies on the mechanism of specificity of human natural killer cells for tumor cells: correlation between target cell transferrin receptor expression and competitive activity. Cell Immunol. 1985 Dec;96(2):255–266. doi: 10.1016/0008-8749(85)90358-2. [DOI] [PubMed] [Google Scholar]
- López-Botet M., Silva A., Rodríguez J., de Landazuri M. O. Generation of T cell blasts with NK-like activity in human MLC: cellular precursors, IL 2 responsiveness, and phenotype expression. J Immunol. 1982 Sep;129(3):1109–1115. [PubMed] [Google Scholar]
- MORGAN E. H. PASSAGE OF TRANSFERRIN, ALBUMIN AND GAMMA GLOBULIN FROM MATERNAL PLASMA TO FOETUS IN THE RAT AND RABBIT. J Physiol. 1964 May;171:26–41. doi: 10.1113/jphysiol.1964.sp007359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan K. F., Hartnett M. E., Matis S. A., Winkelstein A., Abo T. Natural killer cells suppress human erythroid stem cell proliferation in vitro. Blood. 1984 Feb;63(2):260–269. [PubMed] [Google Scholar]
- Mattia E., Rao K., Shapiro D. S., Sussman H. H., Klausner R. D. Biosynthetic regulation of the human transferrin receptor by desferrioxamine in K562 cells. J Biol Chem. 1984 Mar 10;259(5):2689–2692. [PubMed] [Google Scholar]
- Newman R. A., Warner J. F., Dennert G. NK recognition of target structures: is the transferrin receptor the NK target structure? J Immunol. 1984 Oct;133(4):1841–1845. [PubMed] [Google Scholar]
- ODA M., PUCK T. T. The interaction of mammalian cells with antibodies. I. J Exp Med. 1961 Mar 1;113:599–610. doi: 10.1084/jem.113.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med. 1986 Sep 1;164(3):814–825. doi: 10.1084/jem.164.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. L., Azari P. Effect of iron transferrin on nucleic acid synthesis in phytohemagglutinin-stimulated human lymphocytes. Cell Immunol. 1975 Jan;15(1):94–99. doi: 10.1016/0008-8749(75)90167-7. [DOI] [PubMed] [Google Scholar]
- Reichard P., Ehrenberg A. Ribonucleotide reductase--a radical enzyme. Science. 1983 Aug 5;221(4610):514–519. doi: 10.1126/science.6306767. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Pross H. F. The biology of the human natural killer cell. J Clin Immunol. 1982 Oct;2(4):249–263. doi: 10.1007/BF00915064. [DOI] [PubMed] [Google Scholar]
- Salmon M., Bacon P. A. Abnormal populations of activated lymphocytes in the rheumatoid one-way MLR. Agents Actions. 1986 Dec;19(5-6):269–270. doi: 10.1007/BF01971225. [DOI] [PubMed] [Google Scholar]
- Salmon M., Bacon P. A., Symmons D. P., Walton K. W. Transferrin receptor expression by stimulated cells in mixed lymphocyte culture. Immunology. 1985 Mar;54(3):559–564. [PMC free article] [PubMed] [Google Scholar]
- Tartof D., Yung C. W., Curran J. J., Livingston C., Thalji Z. Cells that mediate NK like cytotoxicity are present in the human delayed type hypersensitivity response. Clin Exp Immunol. 1984 Nov;58(2):462–469. [PMC free article] [PubMed] [Google Scholar]
- Thelander L., Gräslund A., Thelander M. Continual presence of oxygen and iron required for mammalian ribonucleotide reduction: possible regulation mechanism. Biochem Biophys Res Commun. 1983 Feb 10;110(3):859–865. doi: 10.1016/0006-291x(83)91040-9. [DOI] [PubMed] [Google Scholar]
- Vodinelich L., Sutherland R., Schneider C., Newman R., Greaves M. Receptor for transferrin may be a "target" structure for natural killer cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):835–839. doi: 10.1073/pnas.80.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vánky F. T., Argov S. A., Einhorn S. A., Klein E. Role of alloantigens in natural killing. Allogeneic but not autologous tumor biopsy cells are sensitive for interferon-induced cytotoxicity of human blood lymphcoytes. J Exp Med. 1980 May 1;151(5):1151–1165. doi: 10.1084/jem.151.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martino M., Rossi M. E., Muccioli A. T., Resti M., Vierucci A. Interference of hepatitis B virus surface antigen with natural killer cell function. Clin Exp Immunol. 1985 Jul;61(1):90–95. [PMC free article] [PubMed] [Google Scholar]



