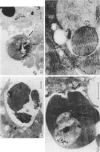
Abstract
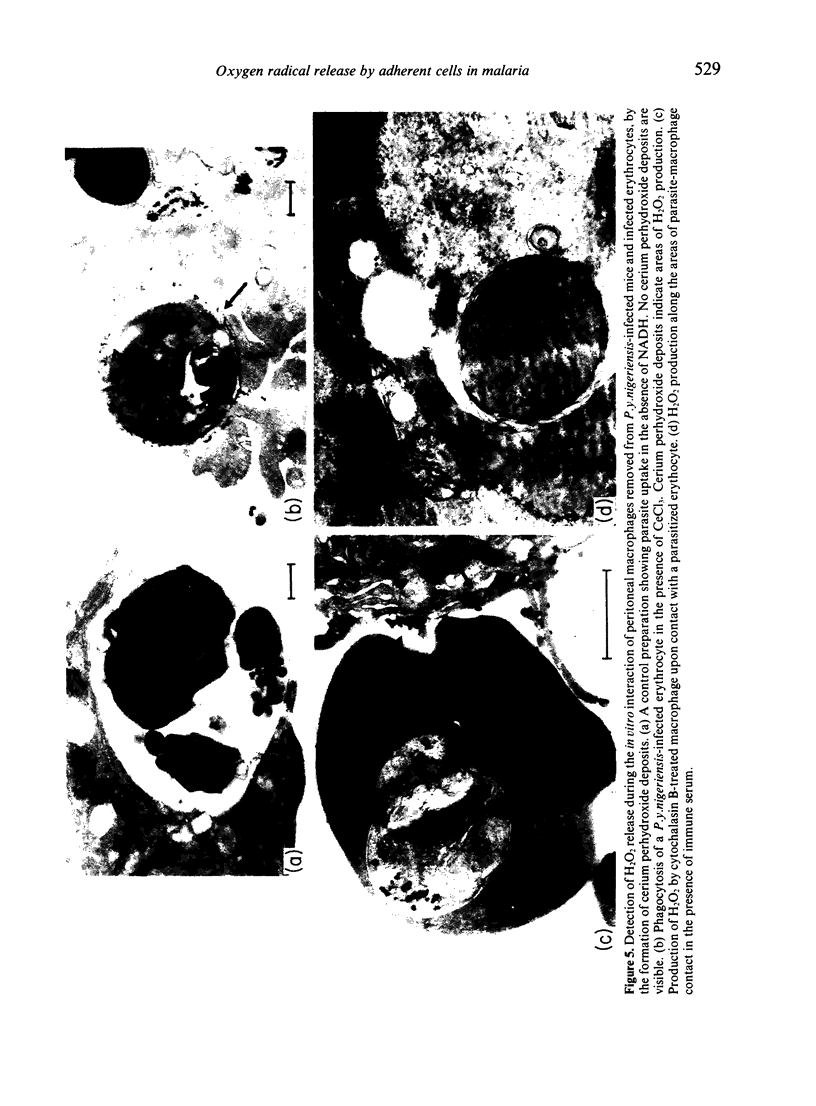
A series of experiments was carried out to assess the levels of reactive oxygen intermediates (ROI) released by macrophages and monocytes during an acute malarial infection, and to consider the importance of oxidant-induced parasite killing in host protection. Adherent cell populations were removed from the peritoneum and spleen of BALB/c and B10/D2/n mice between Days 0-5 of a Plasmodium yoelii nigeriensis infection. These cell populations were quantified, characterized and their ROI-releasing capacity was measured by following ferricytochrome c reduction upon stimulation with phorbol myristate acetate (PMA). Both strains of mice displayed higher numbers of macrophages and macrophage precursors as the infection progressed; this rise was more marked and accompanied by splenomegaly in BALB/c mice. A concurrent decrease in peritoneal cell numbers was observed. Splenic adherent cell populations released much lower levels of ROI than peritoneal macrophages upon triggering. The levels of ROI released from BALB/c splenic adherent cells rose gradually until Day 3, when the parasitaemia was slightly decreased. In contrast, splenic populations from B10 mice had a decreased capacity to release ROI, particularly after Day 3, when the parasitaemia rose sharply. In further studies, electron microscopy was used to detect H2O2 release during the in vitro interaction of peritoneal macrophages and parasitized erythrocytes. Cerium chloride staining techniques demonstrated that H2O production was not dependent on phagocytosis or the presence of immune serum, although levels were increased by the presence of the latter.
Full text
PDF


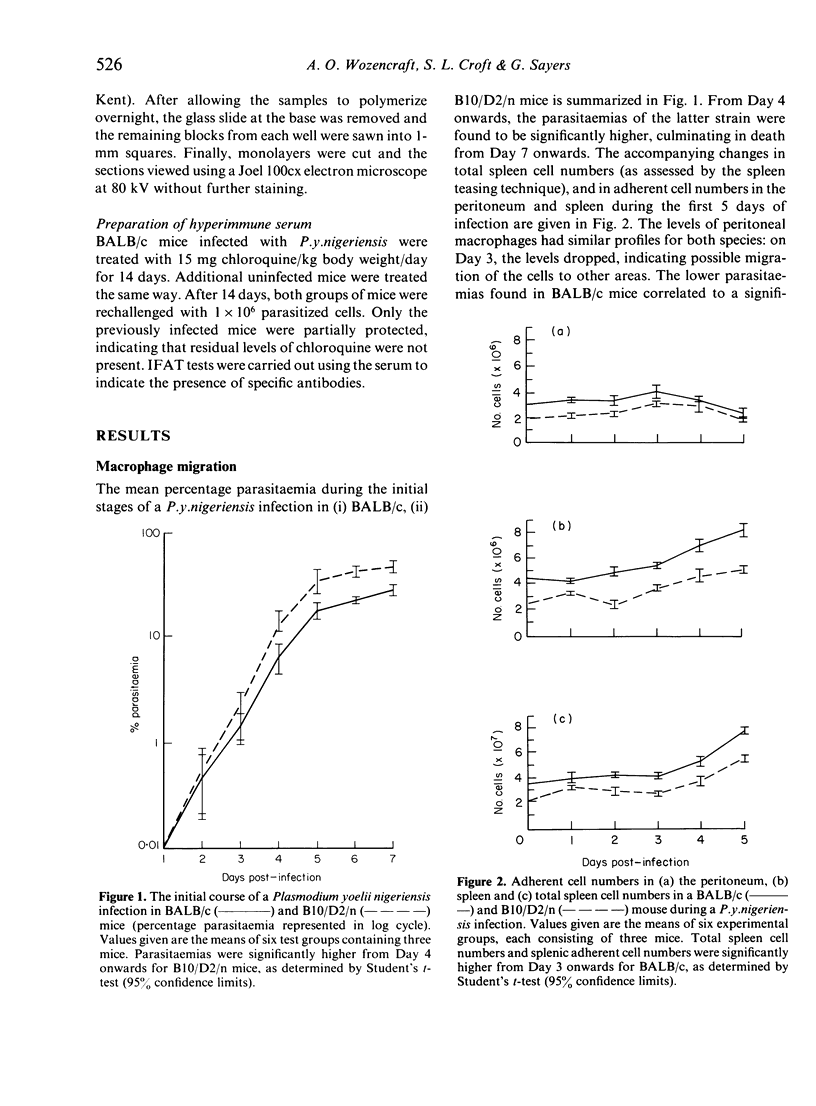
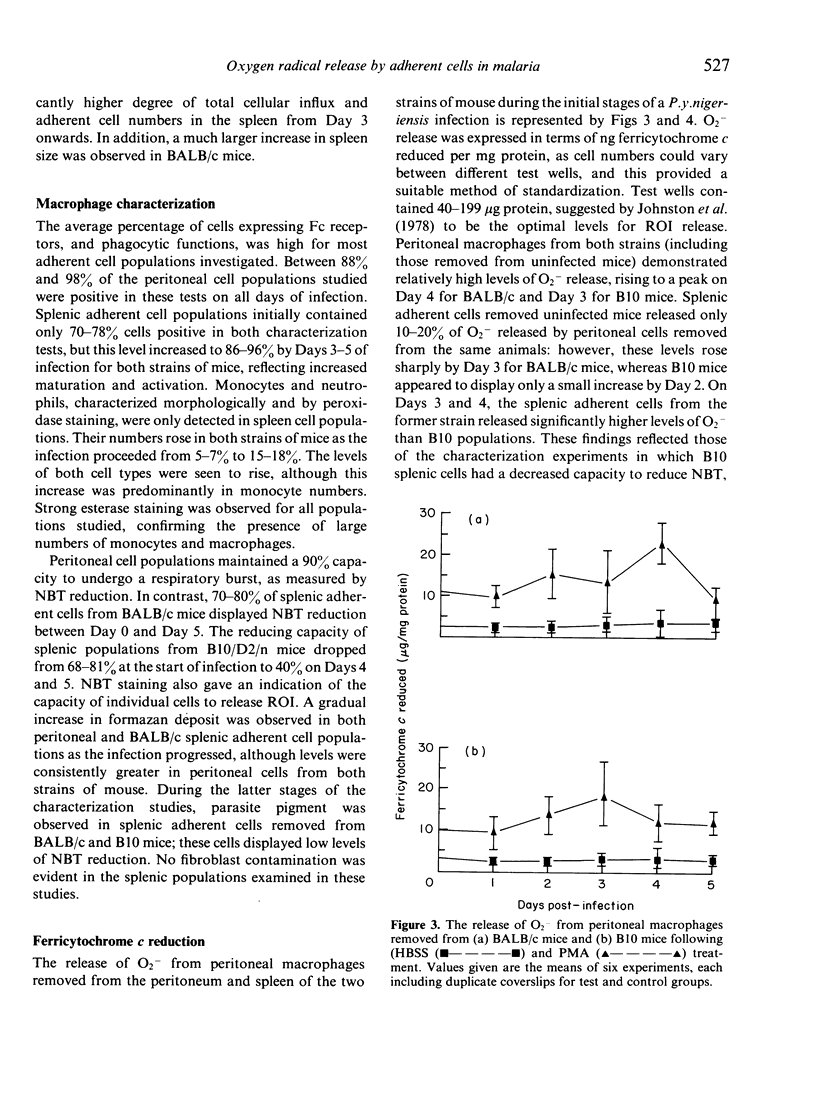
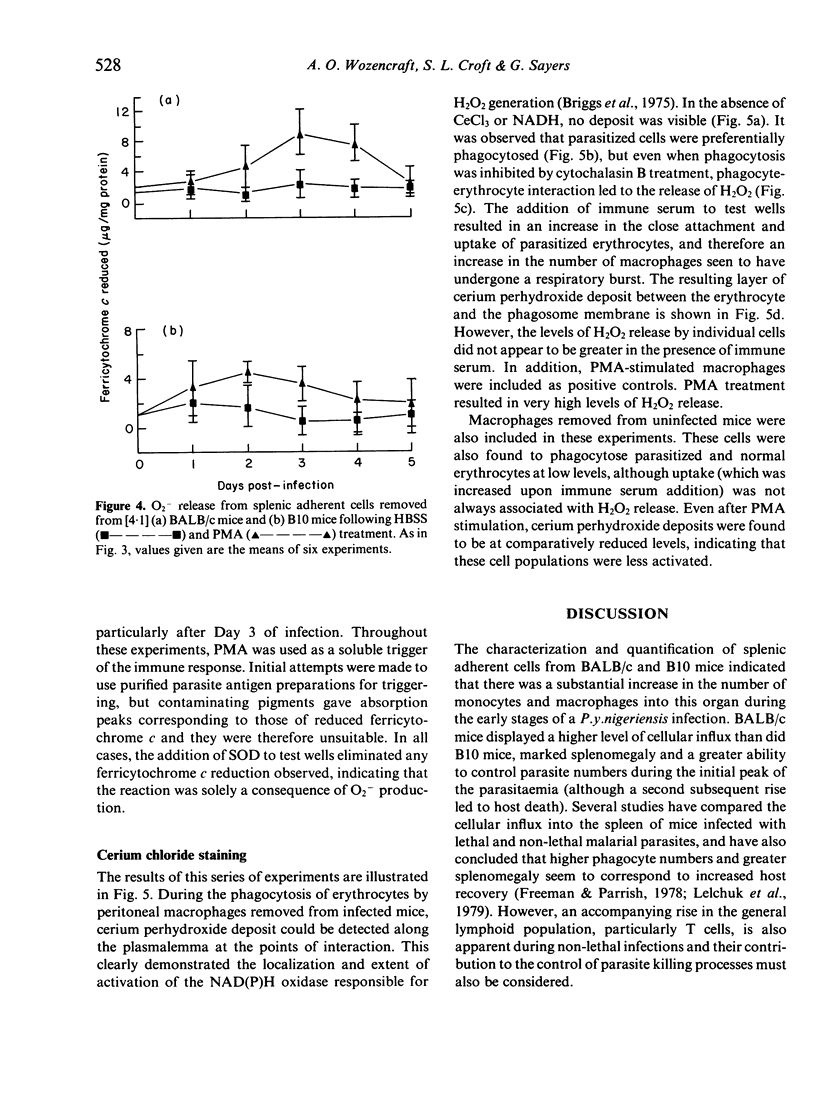


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Eugui E. M. The role of cell-mediated immune responses in resistance to malaria, with special reference to oxidant stress. Annu Rev Immunol. 1983;1:361–392. doi: 10.1146/annurev.iy.01.040183.002045. [DOI] [PubMed] [Google Scholar]
- Berton G., Gordon S. Regulation of superoxide anion release by mouse macrophages in culture. Trans R Soc Trop Med Hyg. 1983;77(5):610–613. doi: 10.1016/0035-9203(83)90188-8. [DOI] [PubMed] [Google Scholar]
- Berton G., Gordon S. Superoxide release by peritoneal and bone marrow-derived mouse macrophages. Modulation by adherence and cell activation. Immunology. 1983 Aug;49(4):693–704. [PMC free article] [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V., Kaufmann S. H., Simon M. M., Fischer H. Role of macrophages in malaria: O2 metabolite production and phagocytosis by splenic macrophages during lethal Plasmodium berghei and self-limiting Plasmodium yoelii infection in mice. Infect Immun. 1984 Jun;44(3):743–746. doi: 10.1128/iai.44.3.743-746.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada A., Cruchaud A., Perrin L. H. Phagocytosis of Plasmodium falciparum-parasitized erythrocytes by human polymorphonuclear leukocytes. J Parasitol. 1983 Feb;69(1):49–53. [PubMed] [Google Scholar]
- Crofton R. W., Diesselhoff-den Dulk M. M., van Furth R. The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J Exp Med. 1978 Jul 1;148(1):1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., Playfair J. H. Killing of Plasmodium yoelii by enzyme-induced products of the oxidative burst. Infect Immun. 1984 Feb;43(2):451–456. doi: 10.1128/iai.43.2.451-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. R., Parish C. R. Spleen cell changes during fatal and self-limiting malarial infections of mice. Immunology. 1978 Sep;35(3):479–484. [PMC free article] [PubMed] [Google Scholar]
- Friedman M. J. Oxidant damage mediates variant red cell resistance to malaria. Nature. 1979 Jul 19;280(5719):245–247. doi: 10.1038/280245a0. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A., Sinden R. E. The purification of gametocytes of Plasmodium falciparum and P. yoelii nigeriensis by colloidal silica (Percoll) gradient centrifugation. Trans R Soc Trop Med Hyg. 1982;76(4):503–509. doi: 10.1016/0035-9203(82)90150-x. [DOI] [PubMed] [Google Scholar]
- Lelchuk R., Dockrell H. M., Playfair J. H. T-independent macrophage changes in murine malaria. Clin Exp Immunol. 1983 Mar;51(3):487–493. [PMC free article] [PubMed] [Google Scholar]
- Lelchuk R., Taverne J., Agomo P. U., Playfair J. H. Development and suppression of a population of late-adhering macrophages in mouse malaria. Parasite Immunol. 1979 Spring;1(1):61–78. doi: 10.1111/j.1365-3024.1979.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Schulman S., Shear H. L. Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol. 1984 Sep;133(3):1601–1608. [PubMed] [Google Scholar]
- Ockenhouse C. F., Shear H. L. Oxidative killing of the intraerythrocytic malaria parasite Plasmodium yoelii by activated macrophages. J Immunol. 1984 Jan;132(1):424–431. [PubMed] [Google Scholar]
- Page D. T., Garvey J. S. Isolation and characterization of hepatocytes and Kupffer cells. J Immunol Methods. 1979;27(2):159–173. doi: 10.1016/0022-1759(79)90262-x. [DOI] [PubMed] [Google Scholar]
- Ward K. N., Warrell M. J., Rhodes J., Looareesuwan S., White N. J. Altered expression of human monocyte Fc receptors in Plasmodium falciparum malaria. Infect Immun. 1984 Jun;44(3):623–626. doi: 10.1128/iai.44.3.623-626.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozencraft A. O., Dockrell H. M., Taverne J., Targett G. A., Playfair J. H. Killing of human malaria parasites by macrophage secretory products. Infect Immun. 1984 Feb;43(2):664–669. doi: 10.1128/iai.43.2.664-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]