Abstract
Oral treponemes are considered to be important in the development and progression of periodontal diseases. We investigated the mechanisms of recognition and activation of human gingival epithelial cells (HGEC) with the oral treponemes Treponema denticola, Treponema vincentii, and Treponema medium and their outer membrane extracts (OMEs). T. vincentii and T. medium but not T. denticola produced interleukin 8 (IL-8) in an HGEC culture. Further, all three treponemes induced IL-8 mRNA expression and NF-κB activation in HGEC. Among them, T. denticola especially exhibited trypsin- and chymotrypsin-like protease activities, and the addition of chymostatin, a chymotrypsin protease inhibitor, resulted in detectable IL-8 production by HGEC cultured with T. denticola. Additionally, IL-8 mRNA expression in HGEC cultured with the three treponemes and their OMEs was definitely inhibited by the mouse anti-human Toll-like receptor 2 (TLR2) monoclonal antibody TL2.1. These findings suggest that oral treponemes and their OMEs activate HGEC through TLR2.
Periodontal diseases are clinically defined as inflammation of gingival tissues followed by destruction of periodontal ligaments and alveolar bone, processes which are the result of augmentation of numerous bacterial species in subgingival plaque. Among these bacteria, oral treponemes and gram-negative, anaerobic, motile, and helical rods are generally isolated from patients with periodontal diseases (15); in contrast, no or only a few treponemes are found in healthy people (31). These oral treponemes have been revealed to be closely associated with various types of periodontal diseases (10, 13, 42). Further, human immunodeficiency virus-positive people with gingivitis or adult periodontitis have been shown to have increased numbers of oral treponemes in subgingival plaque (41, 45). Among them, Treponema denticola and Treponema vincentii have been observed to adhere to and invade connective tissues as well as gingival epithelial cells (9, 27, 39, 54), and T. denticola has also been shown to induce cytokine production from human gingival fibroblasts (34). Further, Asai et al. recently examined the frequency of occurrence of oral treponemes in subgingival plaque samples using real-time PCR assays and found that the numbers of T. denticola and T. medium organisms were increased in plaque samples from deep periodontal pockets, whereas T. vincentii was mainly found in shallow pockets (3).
Various cytokines have been shown to play important roles in the pathogenesis of periodontal diseases (43, 58). Among them, interleukin 8 (IL-8), a CXC chemokine produced by gingival epithelial cells, is considered to be an important factor that initiates inflammatory reactions in gingival tissues (24). IL-8 has been detected in the gingival tissues of patients with periodontal diseases, and the level of IL-8 mRNA has also been demonstrated to correspond to the severity of periodontal diseases (53).
Human gingival epithelial cells (HGEC) play an important role as the first barrier against periodontopathic bacteria and their metabolic products (25). Asai et al. previously established immortalized epithelial cell lines, derived from human gingival tissues, in order to study their interactions with periodontopathic bacteria (4). The Toll-like receptor (TLR) family, a large family with extracellular leucine-rich repeats and a cytoplasmic Toll-IL-1 receptor homology domain and for which 10 members (TLR1 to TLR10) have been reported, is known to play an important role in the recognition of structurally conserved pathogen-associated microbial products (4, 11, 12, 17, 36, 37, 44, 50, 57). In the present study, we attempted to demonstrate the profiles of expression of all of the known TLRs and their related molecules from HGEC as well as the mechanisms of recognition and activation of these cells with the oral treponemes T. denticola, T. vincentii, and T. medium and their outer membrane extracts (OMEs).
MATERIALS AND METHODS
Bacterial cultures and preparation of OMEs.
T. denticola ATCC 35404, T. vincentii ATCC 35580, and T. medium ATCC 700293 were grown anaerobically in Trypticase-yeast extract-gelatin-volatile fatty acids-serum (TYGVS) broth containing 5% rabbit serum (Gibco Laboratories, Grand Island, N.Y.) at 37°C for 72 h (55). Bacterial cell counts were estimated by using phase-contrast microscopy and a Petroff-Hausser bacterial counter (Hausser and Son, Philadelphia, Pa.). For the analyses, the bacteria were centrifuged at 1,500 × g for 20 min and then washed three times with phosphate-buffered saline (PBS; Sigma Chemical Co., St. Louis, Mo.). The bacterial cells were resuspended and serially diluted with pyrogen-free cell culture medium.
The oral treponeme OMEs were prepared as described previously (28). Briefly, the bacterial cells were harvested during the late stationary phase, washed, resuspended in PBS containing 10 mM MgCl2, and extracted in Triton X-100. After repeated centrifugation, the supernatant was dialyzed for several days until the outer membrane was precipitated, and then centrifugation at 25,000 × g was performed. The pellet was resuspended in distilled water and stored at −20°C until use. The dry weight of the extract was determined after freeze-drying. For all experiments, the OMEs were dissolved in PBS and serially diluted with pyrogen-free cell culture medium. Endotoxicity could not be detected in these preparations by a colorimetric Limulus amoebocyte lysate assay (Seikagaku Co., Tokyo, Japan).
Reagents.
Mouse anti-human TLR2 monoclonal antibody TL2.1 was purchased from Cascade Bioscience Inc. (Winchester, Miss.). Mouse immunoglobulin G2a (IgG2a; Dako, Glostrup, Denmark) was used as an isotype control for TL2.1. Phorbol 12-myristate 13-acetate (PMA) was obtained from Sigma. Chymostatin (Roche, Indianapolis, Ind.) was dissolved at 1.5 mg/ml in dimethyl sulfoxide and then serially diluted with pyrogen-free cell culture medium as described below.
Cells.
The generation of the human papillomavirus 16 E6- and E7-immortalized HGEC lines HGEC-1 and HGEC-2 has been described elsewhere (4). Other cell lines, HGEC-3 and HGEC-4, from normal human gingival tissues (ca. 500 mg) obtained from two patients who required tooth extraction for reasons other than periodontal disease after receiving informed consent, were also established. These cell lines were subsequently maintained in a long-term culture with HuMedia-KG2 (Kurabo Biomedicals, Osaka, Japan) and used between passages 50 and 60. Human intestinal epithelial cell line Caco-2 was obtained from Dainippon Pharmaceutical (Osaka, Japan) and maintained in Eagle's minimum essential medium (Sigma) supplemented with 10% fetal bovine serum (Sigma) and 0.1 mM nonessential amino acids (Gibco). Heparinized venous blood was drawn from healthy donors and subjected to fractionation with Histopaque-1077 (Sigma) to obtain human peripheral blood mononuclear cells (PBMC) (6).
RT-PCR.
Total cellular RNAs were extracted from HGEC and PBMC with RNAzolB (Tel-Test, Friendswood, Tex.) according to the manufacturer's instructions and then treated with RNase-free DNase (Takara Biochemicals, Shiga, Japan) as described previously (16). Reverse transcription (RT) and PCR were conducted by using avian myeloblastosis virus reverse transcriptase (TaKaRa Biochemicals) and Taq polymerase (TaKaRa), respectively. PCR assays were conducted for 30 cycles with a TaKaRa Thermal Cycler MP by using the primer pairs and conditions described in Table 1. For the detection of IL-8 and IL-6 mRNAs in HGEC-1, total cellular RNAs were extracted after HGEC-1 were cultured with 5 × 107 cells of the oral treponemes or 1 μg of OMEs/ml for 2 h. In some experiments, HGEC-1 were incubated with or without 1 μg of TL2.1 or mouse IgG2a/ml for 30 min at room temperature before the addition of the oral treponemes or their OMEs. As a negative control, non-reverse-transcribed samples were amplified by PCR. Following PCR, 10 μl of the total amplified product was electrophoresed on ethidium bromide-stained 1% agarose gels and visualized under UV fluorescence.
TABLE 1.
PCR primer pairs used for amplification of human mRNA
| Product | Primer | Orientation | Annealing temp (°C) | Product size (bp) | GenBank accession no. |
|---|---|---|---|---|---|
| TLR1 | CACCAAGTTGTCAGCGATGT | Forward | 56 | 550 | U88540 |
| CCACATCCAGGAAGGTCAGT | Reverse | ||||
| TLR2 | GCCAAAGTCTTGATTGATTGG | Forward | 53 | 349 | U88878 |
| TTGAAGTTCTCCAGCTCCTG | Reverse | ||||
| TLR3 | AGTGCCCCCTTTGAACTCTT | Forward | 56 | 546 | U88879 |
| GCCAGTTCAAGATGCAGTGA | Reverse | ||||
| TLR4 | GGTGGAAGTTGAACGAATGG | Forward | 56 | 598 | U88880 |
| CTGTCCTCCCACTCCAGGTA | Reverse | ||||
| TLR5 | CCTTACAGCGAACCTCATCC | Forward | 56 | 602 | AB060695 |
| AAGAGGGAAACCCCAGAGAA | Reverse | ||||
| TLR6 | GTGAGTGGTGCCATTACGAA | Forward | 56 | 551 | AB020807 |
| TTTGGGAAAGCAGAGTGGAG | Reverse | ||||
| TLR7 | GGCTCTGTGGGAGTTCTGTC | Forward | 54 | 631 | AF240467 |
| TGCTGGGATTACAAGCATGA | Reverse | ||||
| TLR8 | TCCTTCAGTCGTCAATGCTG | Forward | 56 | 662 | AF246971 |
| GTAGGGAGCTTGGCAGTTTG | Reverse | ||||
| TLR9 | CAGCAGCTCTGCAGTACGTC | Forward | 58 | 555 | AB045180 |
| CCTCCAGCAGGAAGTCCATA | Reverse | ||||
| TLR10 | GGATGCTAGGTCAATGCACA | Forward | 56 | 504 | AF296673 |
| ATAGCAGCTCGAAGGTTTGC | Reverse | ||||
| MyD88 | TCTTTCACACCTCCCAGCTT | Forward | 56 | 582 | U70451 |
| GGTACATTGGGTCCTTTCCA | Reverse | ||||
| RP105 | AATCAGTGCTGCCAATTTCC | Forward | 57 | 286 | D83597 |
| GTAAGCGGGTAAATGCCAAA | Reverse | ||||
| MD-1 | TCCTATCCCATCTGTGAGGC | Forward | 57 | 382 | AF057178 |
| ATCTGTGGAGTCTGGGGATG | Reverse | ||||
| MD-2 | AGGGGCACGAGGTAAATCTT | Forward | 56 | 526 | AB018549 |
| GGCTCCCAGAAATAGCTTCA | Reverse | ||||
| CD14 | AGGACTTGCACTTTCCAGCTTG | Forward | 59 | 568 | M86511 |
| TCCCGTCCAGTGTCAGGTTATC | Reverse | ||||
| IL-8 | ATGACTTCCAAGCTGGCCGTGGCT | Forward | 60 | 294 | Y00787 |
| TCTCAGCCCTCTTCAAAAACTTCTC | Reverse | ||||
| IL-6 | TCTCAGCCCTGAGAAAGGAGAC | Forward | 60 | 438 | M54894 |
| GAAGAGCCCTCAGGCTGGACTG | Reverse | ||||
| β-Actin | GTGGGCGCCCCAGGCACCA | Forward | 58 | 506 | X00351 |
| CTCCTTAATGTCACGCACGATTTC | Reverse |
Luciferase assay.
HGEC were seeded in a 24-well flat-bottom microtiter plate (Falcon 3047; Becton Dickinson and Co., Lincoln Park, N.J.) in a manner similar to that described above. After incubation for 24 h at 37°C in humidified air containing 5% (vol/vol) CO2, the monolayers were washed three times with PBS and then transfected with 0.8 μg of plasmid pNF-κB-Luc (Stratagene Co., La Jolla, Calif.) by using TransFast transfection reagent (Promega Co., Madison, Wis.). Plasmid pFC-MEKK was used as a positive control plasmid in this assay. After an initial incubation for 24 h, HGEC were incubated with 5 × 107 cells of the oral treponemes in 500 μl of HuMedia-KG2 at 37°C for 4 h. After incubation, luciferase activity was determined by using a luciferase assay substrate (Promega), and luminescence was quantified with a Luminometer (Promega).
Detection of IL-8 production.
A single cell suspension (105 cells per well) was seeded in a 24-well flat-bottom microtiter plate. After incubation for 16 h at 37°C in humidified air containing 5% (vol/vol) CO2, the monolayers were washed three times with PBS. The cells were incubated with various doses of the oral treponemes and their OMEs for various times at 37°C in humidified air containing 5% (vol/vol) CO2; the mixtures then were centrifuged at 12,000 × g for 5 min. In some experiments, HGEC were incubated with or without 1 μg of TL2.1 or mouse IgG2a/ml for 30 min at room temperature before the addition of the test specimens. The supernatants were stored at −80°C until assayed for cytokine production. The production of IL-8 in the culture supernatants was determined by an enzyme-linked immunosorbent assay (ELISA) performed according to the manufacturer's instructions (ELISA kit system; Genzyme-Techne, Minneapolis, Minn.), and the results were analyzed by using a standard curve prepared for each assay.
Analysis of enzymatic activity.
The amounts of cell-associated enzymes in the oral treponemes were determined from cells grown in TYGVS broth as described above. These cells were centrifuged and then suspended in PBS for enzyme analysis. An API ZYM chromogenic assay system (Biomerieux, Marcy-l'Etoile, France) was used for the estimation of enzyme levels, and the intensity of the color reaction was graded semiquantitatively by comparison to a standard API ZYM color reaction chart. Three independent experiments with the different treponemes were performed.
PMA-induced IL-8 degradation.
HGEC were incubated with 10 ng of PMA/ml for 24 h at 37°C in humidified air containing 5% (vol/vol) CO2, and supernatants were collected by centrifugation at 12,000 × g for 5 min. The oral treponemes at various doses were cultured in the same supernatant (1 ml/well) under the same culture conditions. After incubation, IL-8 levels in the supernatants were measured by an ELISA.
Effect of chymostatin on IL-8 degradation.
HGEC were seeded in a 24-well plate in a manner similar to that described above. The oral treponemes were washed three times with or without 30 μg of chymostatin/ml in PBS and then suspended at a density of 107 cells/ml in HuMedia-KG2 with or without 30 μg of chymostatin/ml. HGEC were cultured with 107 cells of the oral treponemes treated or not treated with chymostatin at 37°C for 12 h. After incubation, supernatants were collected, and IL-8 production was measured by an ELISA.
Statistics.
Data were analyzed by a one-way analysis of variance (ANOVA) with the Bonferroni or Dunn method, and the results are presented as the means ± standard errors of the means (SEM). When an individual result is presented, it is representative of at least three independent experiments.
RESULTS
HGEC activation.
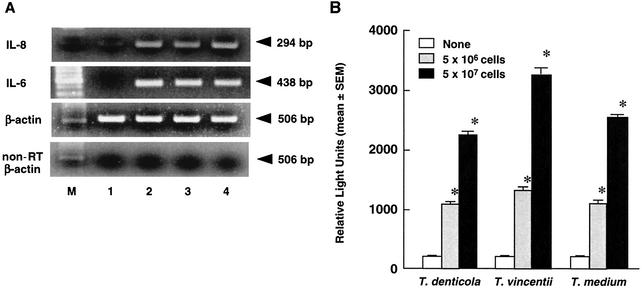
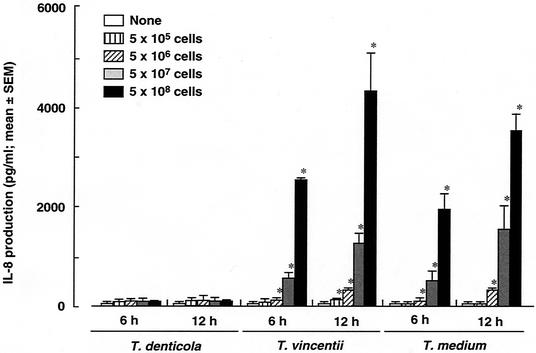
During mucosal infection, gingival epithelial cells are a major source of IL-8, which participates in local inflammation and neutrophil chemotaxis (24). In the present study, IL-8 mRNA expression and NF-κB activation in HGEC cultured with oral treponemes were examined. T. denticola, T. vincentii, and T. medium each clearly induced IL-8 and IL-6 mRNA expression in HGEC (Fig. 1A). These oral treponemes also clearly induced NF-κB activation in a cell-dependent manner (Fig. 1B). Further, we examined IL-8 production by HGEC cultured with various doses of T. denticola, T. vincentii, and T. medium. T. medium and T. vincentii induced significant IL-8 production by HGEC-1 in time- and cell-dependent manners, whereas IL-8 was scarcely found after culturing with T. denticola (Fig. 2). HGEC-2, HGEC-3, and HGEC-4, similar to HGEC-1, were shown to have induced IL-8 and IL-6 mRNA expression and IL-8 production (data not shown).
FIG. 1.
Cytokine mRNA expression and NF-κB activation of HGEC after culturing with oral treponemes. (A) IL-8 and IL-6 mRNAs of HGEC-1 cultured with 5 × 107 cells of oral treponemes for 2 h were analyzed by RT-PCR. β-Actin was assayed as a positive control. PCR products of non-reverse-transcribed (non-RT) samples were examined as a negative control. Lane 1, medium alone; lane 2, T. denticola; lane 3, T. vincentii; lane 4, T. medium; lane M, size marker. Experiments were done at least three times, and representative results are presented. (B) HGEC-1 were transfected with 1 μg of plasmid pNF-κB-Luc and then cultured with the indicated cells of oral treponemes in HuMedia-KG2 at 37°C for 4 h. After incubation, luciferase activity was estimated with a Luminometer. Experiments were done at least three times, and representative results are presented. Each assay was done with triplicate wells, and the data are expressed as the means ± SEM. Asterisks indicate significant differences seen between groups with and without the oral treponemes (P < 0.01).
FIG. 2.
IL-8 production by HGEC after culturing with oral treponemes. HGEC-1 were cultured with the indicated amounts of T. denticola, T. vincentii, or T. medium in HuMedia-KG2 at 37°C for 6 and 12 h. After incubation, supernatants were collected, and IL-8 production was determined by an ELISA. Experiments were done at least three times, and representative results are presented. Each assay was done with triplicate wells, and the data are expressed as the means ± SEM. Asterisks indicate significant differences seen between groups with and without oral treponemes (P < 0.01).
IL-8 degradation.
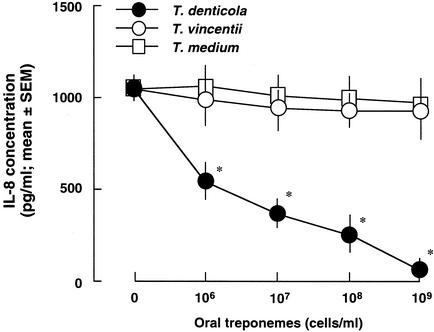
To confirm whether the T. denticola culture was involved in the degradation of IL-8 produced by HGEC, the effect of the oral treponeme culture on PMA induction of IL-8 in HGEC was examined. PMA, an activator of protein kinase C, has been shown to induce IL-8 production in HGEC (46). T. denticola, but not T. vincentii or T. medium, markedly reduced the amount of IL-8 produced by HGEC-1 stimulated with PMA in a cell-dependent manner (Fig. 3).
FIG. 3.
Degradation of PMA-induced IL-8 in HGEC. HGEC-1 were incubated with 10 ng of PMA/ml for 24 h, and supernatants were collected. The indicated doses of oral treponemes were cultured with 1 ml of these supernatants at 37°C for 24 h in humidified air containing 5% (vol/vol) CO2. After incubation, IL-8 production was determined by an ELISA. Experiments were done at least three times, and representative results are presented. Each assay was done with triplicate wells, and the data are expressed as the means ± SEM. Asterisks indicate significant differences seen between groups with and without oral treponemes (P < 0.01).
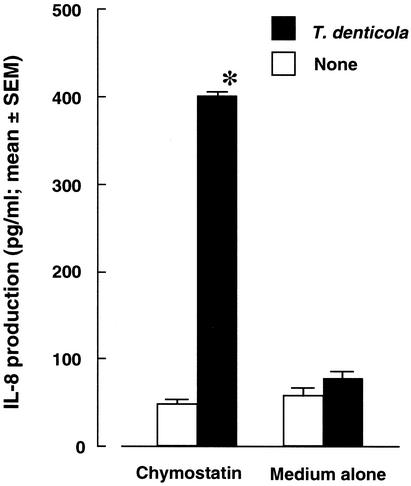
To determine the potential contribution of enzymatic activity of the oral treponemes to virulence expression, we examined the cell-associated enzyme profiles of T. denticola, T. vincentii, and T. medium (Table 2). Notably, T. denticola exhibited high levels of various enzymatic activities, including trypsin-like and chymotrypsin-like protease activities, compared with T. vincentii and T. medium. We consequently examined the inhibitory effect on IL-8 degradation by using chymostatin, a chymotrypsin-like protease inhibitor, and found that it inhibited the reduction by T. denticola of IL-8 expression in HGEC-1 (Fig. 4).
TABLE 2.
Enzymatic activities of oral treponemesa
| Enzyme | Enzyme concn (nmol/5 × 108 cells) for:
|
||
|---|---|---|---|
| T. denticola ATCC 35404 | T. vincentii ATCC 35580 | T. medium ATCC 700293 | |
| Alkaline phosphatase | ≥40 | 0 | 0 |
| Esterase lipase (C8) | 7.5 | 0 | 0 |
| Leucine aminopeptidase | 30 | 7.5 | 5 |
| Valine aminopeptidase | 10 | 0 | 0 |
| Trypsin | ≥40 | 0 | 0 |
| Chymotrypsin | 30 | 0 | 0 |
| Acid phosphatase | ≥40 | 10 | 10 |
| Phosphohydrolase | 7.5 | 5 | 7.5 |
| β-Galactosidase | 10 | ≥40 | ≥40 |
| α-Glucosidase | 30 | 0 | 0 |
| β-Glucosidase | 5 | 0 | 0 |
| N-Acetyl-β-glucosaminidase | 0 | 30 | 0 |
T. denticola, T. vincentii, and T. medium were grown as described in Materials and Methods. All treponemes were centrifuged, and their concentrations were adjusted to 1010 cells/ml. Then, a 65-μl sample containing 5 × 108 cells was added to each cupule of the API ZYM strip. The resulting changes were evaluated in triplicate on different days, and the mean values are presented. The tested treponemes were negative for lipase (C14), cysteine aminopeptidase, α-galactosidase, β-glucuronidase, α-mannosidase, and α-fucosidase.
FIG. 4.
Effect of chymostatin on IL-8 production by HGEC after culturing with T. denticola. Bacterial cells were washed three times with or without 30 μg of chymostatin in PBS/ml and then suspended at a density of 107 cells/ml in HuMedia-KG2 with or without 30 μg of chymostatin/ml. HGEC-1 were cultured with 107 cells of T. denticola treated or not treated with chymostatin at 37°C for 12 h. After incubation, supernatants were collected, and IL-8 production was determined by an ELISA. Experiments were done at least three times, and representative results are presented. Each assay was done with triplicate wells, and the data are expressed as the means ± SEM. Asterisks indicate significant differences seen between groups with and without T. denticola (P < 0.01).
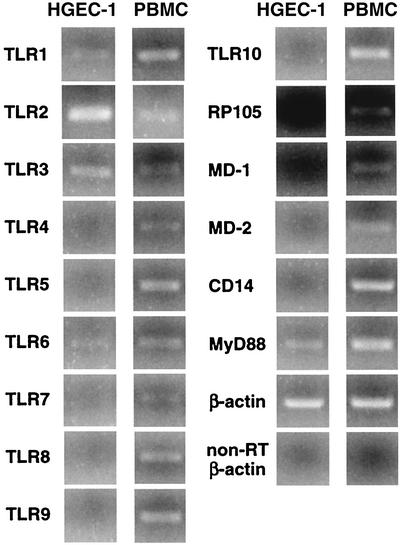
mRNA expression profiles for TLRs and their related molecules in HGEC.
The expression of mRNAs for all known TLRs and their related molecules in HGEC was evaluated by RT-PCR. HGEC-1 expressed mRNAs for TLR1, TLR2, TLR3, TLR6, and MyD88 (Fig. 5) (4); however, mRNAs for TLR4, TLR5, TLR7, TLR8, TLR9, TLR10, RP105, MD-1, MD-2, and CD14 were not detected. A similar pattern of mRNA expression was shown for HGEC-2, HGEC-3, and HGEC-4 (data not shown). Human PBMC, used as a control, also expressed mRNAs for all of the TLRs. RT-PCR analysis of β-actin expression confirmed the quality of all RNA preparations, and no band was detected for the non-reverse-transcribed sample by PCR.
FIG. 5.
TLR mRNA expression of HGEC. The expression of human TLR mRNA was analyzed by RT-PCR as detailed in Materials and Methods. Human PBMC were used as a positive source of TLR mRNA expression to confirm the specificity of the primers and the accuracy of the assay. The β-actin gene was assayed as a positive control. PCR products of non-reverse-transcribed (non-RT) samples were examined as a negative control. M, size marker. Experiments were done at least three times, and representative results are presented.
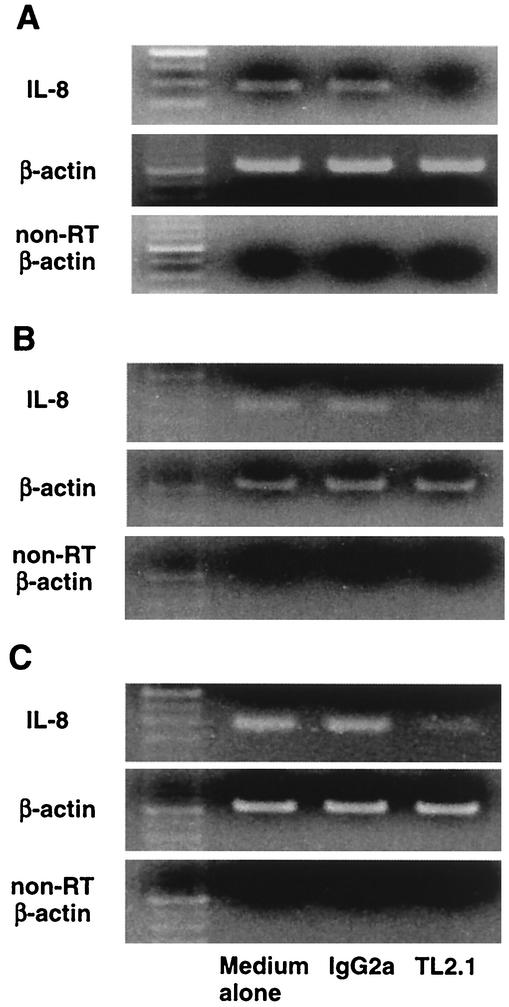
Inhibitory effect of mouse monoclonal antibody to human TLR2 on activation of HGEC cultured with oral treponemes.
To examine the recognition of HGEC in response to oral treponemes, IL-8 mRNA expression in HGEC treated with a mouse monoclonal antibody to human TLR2, TL2.1, or mouse IgG2a as a control before the addition of oral treponemes was examined. TL2.1 significantly inhibited IL-8 mRNA expression induced by the three treponemes in HGEC-1 (Fig. 6). These results indicated that the three treponemes activated HGEC through TLR2.
FIG. 6.
Effect of a mouse anti-human TLR2 monoclonal antibody on the induction of IL-8 mRNA expression in HGEC after stimulation with oral treponemes. HGEC-1 were preincubated with or without a mouse monoclonal antibody to human TLR2 (TL2.1) or mouse IgG2a at 1 μg/ml for 30 min at room temperature and then cultured with 5 × 107 cells of T. denticola (A), T. vincentii (B), or T. medium (C) for 2 h. β-Actin was assayed as a positive control. PCR products of non-reverse-transcribed (non-RT) samples were examined as a negative control. Experiments were done at least three times, and representative results are presented.
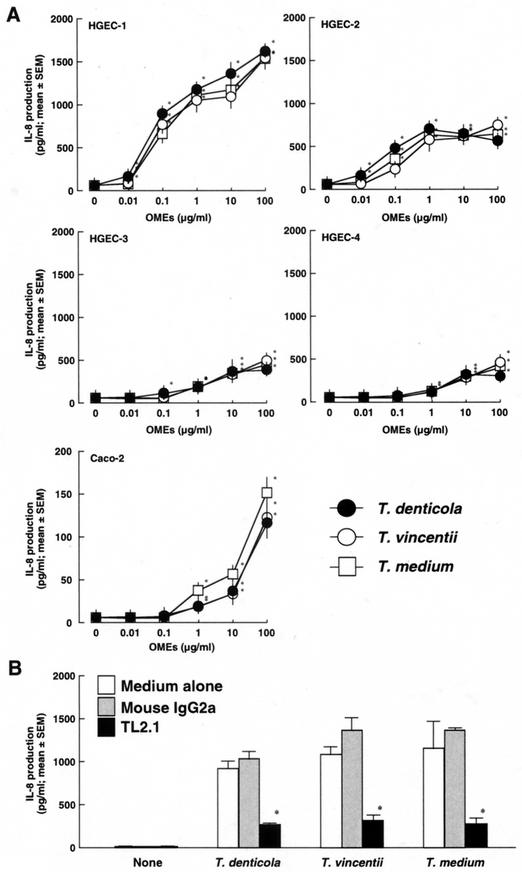
Induction of IL-8 production by HGEC stimulated with OMEs from oral treponemes.
IL-8 production by HGEC stimulated with the OMEs from T. denticola, T. vincentii, and T. medium was examined. HGEC-1, HGEC-2, HGEC-3, and HGEC-4 as well as human intestinal epithelial cell line Caco-2, which is known to express TLR2 protein (8), clearly exhibited IL-8 production stimulated with the OMEs in a dose-dependent manner (Fig. 7A). Further, the IL-8-producing activities of HGEC-1 were clearly inhibited by a mouse monoclonal antibody to human TLR2, TL2.1 (Fig. 7B). These results indicated that the OMEs from the three oral treponemes as well as the bacterial cells activated HGEC through TLR2.
FIG.7.
Induction of IL-8 production by HGEC stimulated with OMEs from oral treponemes. (A) HGEC and Caco-2 cells were stimulated with the indicated doses of the OMEs at 37°C for 12 h. After incubation, supernatants were collected, and IL-8 production was determined by an ELISA. (B) HGEC-1 were incubated with or without a mouse monoclonal antibody to human TLR2 (TL2.1) or mouse IgG2a at 1 μg/ml for 30 min at room temperature and then stimulated with 1 μg of the OMEs/ml for 12 h. After incubation, supernatants were collected, and IL-8 production was determined by an ELISA. Experiments were done at least three times, and representative results are presented. Each assay was done with triplicate wells, and the data are expressed as the means ± SEM. Asterisks indicate significant differences seen between groups with and without the test specimens (P < 0.01).
DISCUSSION
Periodontal tissue is a complex structure comprised of resident cells, such as epithelial cells, fibroblasts, periodontal ligament cells, osteoblasts, and osteoclasts, as well as various types of inflammatory cells, which emigrate from the microvasculature of the gingiva in response to dental plaque accumulation (47). In periodontal diseases, these cells induce the production of various pro- and anti-inflammatory factors in the gingiva, as well as gingival crevicular fluid (5). Gingival epithelial cells are the first to come into contact with various oral bacteria. For this reason, it is considered that the induction of gingival epithelial cell responses after stimulation with various oral bacteria leads to the establishment of periodontal diseases.
Asai et al. previously found that immortalized gingival epithelial cell lines, HGEC-1 and HGEC-2, expressed TLR2 but not CD14 or TLR4 on the cell surfaces and also responded to S. aureus peptidoglycan and muramyl dipeptide, a common component of cell wall peptidoglycan, as TLR2 ligands but not to Escherichia coli-type synthetic lipid A as a TLR4 ligand (4). Porphyromonas gingivalis lipopolysaccharide (LPS) and its lipid A fraction were recognized as utilizing TLR2 (33). Ogawa et al. recently showed that further purified P. gingivalis lipid A and its synthetic counterpart induced cell activation via a TLR4/MD-2-MyD88-dependent pathway and that minor bacterial components separated from P. gingivalis lipid A fractions activated cells utilizing TLR2 (36). HGEC did not respond to further purified P. gingivalis 381 lipid A and its synthetic compound (compound PG-381), which is similar to the results obtained for compound 506 (unpublished observations).
It was also shown that epithelial cells from normal human vagina, ectocervix, and endocervix tissues expressed mRNAs for TLR1, TLR2, TLR3, TLR5, and TLR6. However, mRNA for TLR4, which plays an important role as a receptor for bacterial LPS and its active center, lipid A, and mRNA for MD-2, a small secreted protein associated with TLR4 on the surface of innate immune cells, were not expressed (18, 23, 40, 48). A TLR-related protein, RP105, has been identified in B cells and shown to act as both an LPS sensor and a regulator of B-cell proliferation (35). RP105, like TLR4, also requires an MD-2-related protein, MD-1, for its surface expression (32). Further, TLR2 is essential for the signaling of various bacterial components, such as Staphylococcus aureus peptidoglycan (49) and its bioactive muramyl dipeptide (4), bacterial lipoprotein (2, 7), lipoteichoic acid (29), zymosan (56), and P. gingivalis fimbriae (4, 37). In addition, TLR3, TLR5, TLR7, and TLR9 are known to recognize double-stranded viral RNA, bacterial flagellin, imidazoquinoline compound imiquimod and R-848, and bacterial CpG DNA, respectively (1, 20-22). In the present study, the expression of mRNAs for the known TLRs, RP105, and several other proteins in HGEC was examined, and the cells failed to express mRNAs for TLR4, RP105, MD-1, MD-2, or CD14 (Fig. 5) (4). These results indicate that HGEC-1 lack known whole molecules for the recognition and activation of bacterial LPS and its active center, lipid A.
In the present study, we observed IL-8 production in HGEC cultured with T. vincentii and T. medium but not with T. denticola (Fig. 2). It was previously shown that KB cells, a human oral epithelial cell line, cultured with T. denticola expressed no IL-8 production (14). On the other hand, T. denticola, T. vincentii, and T. medium each clearly induced IL-8 mRNA expression as well as NF-κB activation in HGEC in the present study (Fig. 1). Further, the OMEs from these oral treponemes also induced IL-8 production in HGEC (Fig. 7A).
In previous studies, P. gingivalis exhibited a high level of protease activity, after which the protease seemed to reduce IL-8 production by gingival epithelial cells (59), while T. denticola showed highly proteolytic activities (19, 26) and its chymotrypsin-like proteases induced cytopathic activities, such as loosening of cell contacts, collapse of intercellular spaces, and an increase in the permeability of epithelial cells (54). Further, stimulation of a dentilisin protease mutant of T. denticola resulted in an increase in IL-8 production in KB cells (14). In the present study, T. denticola exhibited a variety of protease activities, compared with T. vincentii and T. medium (Table 2); these activities resulted in a decrease in IL-8 production in HGEC induced by PMA (Fig. 3). Further, the increase in IL-8 production induced in HGEC by T. denticola was also seen with additional chymostatin (Fig. 4). These results suggest that a chymotrypsin-like protease from T. denticola degrades exogenous IL-8 production induced by HGEC, an activity which may play an important role in the defense against host immune defenses.
In the present study, we examined the activation of HGEC against three oral treponemes. TLR2 has been shown to be a signal transducer of lipoprotein and lipopeptide from Treponema pallidum, which is associated with syphilis (30). Further, it has also been shown that the glycolipid from Treponema maltophilum, associated with periodontitis in humans, and that of Treponema brennaborense, found in bovine cattle, induce NF-κB activation through TLR2 (38). In our study, a mouse anti-human TLR2 monoclonal antibody, TL2.1, clearly inhibited IL-8 mRNA expression and IL-8 production in HGEC cultured with the three oral treponemes and their OMEs (Fig. 6 and 7B). Human intestinal epithelial cell lines Caco-2 and T84 also have been demonstrated to express TLR2 (8); the OMEs induced IL-8-producing activities in Caco-2 cells (Fig. 7A). These findings suggested that oral treponemes and their OMEs activated other types of epithelial cells expressing TLR2. TLR1 and TLR6 have been shown to associate with TLR2 and to recognize mycoplasmal lipopeptide and mycobacterial lipoprotein along with TLR2, respectively (51, 52). As HGEC expressed mRNAs for TLR1 and TLR6 (Fig. 1), TLR1 and TLR6 may be associated with the recognition of oral treponemes and their OMEs by TLR2. Taken together, the results show that the oral treponemes activate HGEC through TLR2, followed by the induction of NF-κB activation, IL-8 mRNA expression, and IL-8 production. T. denticola, which was distinct from T. vincentii and T. medium in the present study, possesses highly proteolytic activities, which produce enzymes that degrade the exogenous IL-8 produced by HGEC.
Acknowledgments
This study was supported in part by a grant-in-aid for scientific research (B) from the Japan Society for the Promotion of Science (no. 13470390).
Editor: T. R. Kozel
REFERENCES
- 1.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 3.Asai, Y., T. Jinno, H. Igarashi, Y. Ohyama, and T. Ogawa. 2002. Detection and quantification of oral treponemes in subgingival plaque by real-time PCR. J. Clin. Microbiol. 40:3334-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asai, Y., Y. Ohyama, K. Gen, and T. Ogawa. 2001. Bacterial fimbriae and their peptides activate human gingival epithelial cells through Toll-like receptor 2. Infect. Immun. 69:7387-7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartold, P. M., L. J. Walsh, and A. S. Narayanan. 2000. Molecular and cell biology of the gingiva. Periodontology 24:28-55. [DOI] [PubMed] [Google Scholar]
- 6.Böyum, A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Investig. 97:77-89. [PubMed] [Google Scholar]
- 7.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 8.Cario, E., I. M. Rosenberg, S. L. Brandwein, P. L. Beck, H.-C. Reinecker, and D. K. Podolsky. 2000. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164:966-972. [DOI] [PubMed] [Google Scholar]
- 9.Carranza, N., G. R. Riviere, K. S. Smith, D. F. Adams, and T. Maier. 1997. Differential attachment of oral treponemes to monolayers of epithelial cells. J. Periodontol. 68:1010-1018. [DOI] [PubMed] [Google Scholar]
- 10.Chan, E. C., and R. McLaughlin. 2000. Taxonomy and virulence of oral spirochetes. Oral Microbiol. Immunol. 15:1-9. [DOI] [PubMed] [Google Scholar]
- 11.Chuang, T. H., and R. J. Ulevitch. 2001. Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells. Biochim. Biophys. Acta 1518:157-161. [DOI] [PubMed] [Google Scholar]
- 12.Chuang, T. H., and R. J. Ulevitch. 2000. Cloning and characterization of a subfamily of human Toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur. Cytokine Netw. 11:372-378. [PubMed] [Google Scholar]
- 13.Courtois, G. J., III, C. M. Cobb, and W. J. Killoy. 1983. Acute necrotizing ulcerative gingivitis. A transmission electron microscope study. J. Periodontol. 54:671-679. [DOI] [PubMed] [Google Scholar]
- 14.Deng, Q. D., Y. Han, X. Xia, and H. K. Kuramitsu. 2001. Effects of the oral spirochete Treponema denticola on interleukin-8 expression from epithelial cells. Oral Microbiol. Immunol. 16:185-187. [DOI] [PubMed] [Google Scholar]
- 15.Dewhirst, F. E., M. A. Ericson, C. N. Lau, V. A. Levanos, S. K. Boches, J. L. Galvin, and B. J. Paster. 2000. The diversity of periodontal spirochetes by 16S rRNA analysis. Oral Microbiol. Immunol. 15:196-202. [DOI] [PubMed] [Google Scholar]
- 16.Dilworth, D., and J. R. McCarrey. 1992. Single-step elimination of contaminating DNA prior to reverse transcriptase PCR. PCR Methods Appl. 1:279-282. [DOI] [PubMed] [Google Scholar]
- 17.Du, X., Y. Poltrak, Y. Wei, and B. Beutler. 2000. Three novel mammalian Toll-like receptors: gene structure, expression, and evolution. Eur. Cytokine Netw. 11:362-371. [PubMed] [Google Scholar]
- 18.Fichorova, R. N., A. O. Cronin, E. Lien, D. J. Anderson, and R. R. Ingalls. 2002. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of Toll-like receptor 4-mediated signaling. J. Immunol. 168:2424-2432. [DOI] [PubMed] [Google Scholar]
- 19.Grenier, D., V. J. Uitto, and B. C. McBridge. 1990. Cellular location of a Treponemea denticola chymotrypsinlike protease and importance of the protease in migration through the basement membrane. Infect. Immun. 58:347-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 21.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 22.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196-200. [DOI] [PubMed] [Google Scholar]
- 23.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 24.Huang, G. T.-J., S. K. Haake, J.-W. Kim, and N.-H. Park. 1998. Differential expression of interleukin-8 and intercellular adhesion molecule-1 by human gingival epithelial cells in response to Actinobacillus actinomycetemcomitans or Porphyromonas gingivalis infection. Oral Microbiol. Immunol. 13:301-309. [DOI] [PubMed] [Google Scholar]
- 25.Kagnoff, M. F., and L. Eckmann. 1997. Epithelial cells as sensors for microbial infection. J. Clin. Investig. 100:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kesavalu, L., S. G. Walker, S. C. Holt, R. R. Crawley, and J. L. Ebersole. 1997. Virulence characteristics of oral treponemes in a murine model. Infect. Immun. 65:5096-5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keulers, R. A., J. C. Maltha, F. H. Mikx, and J. M. Wolters-Lutgerhorst. 1993. Attachment of T. denticola strains ATCC 33520, ATCC 35405, B11 and Ny541 to a morphologically distinct population of rat palatal epithelial cells. J. Periodontal Res. 28:274-280. [DOI] [PubMed] [Google Scholar]
- 28.Ko, K. S., M. Glogauer, C. A. McCulloch, and R. P. Ellen. 1998. Treponema denticola outer membrane inhibits calcium flux in gingival fibroblasts. Infect. Immun. 66:703-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehner, M. D., S. Morath, K. S. Michelsen, R. R. Schumann, and T. Hartung. 2001. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J. Immunol. 166:5161-5167. [DOI] [PubMed] [Google Scholar]
- 30.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 31.Loesche, W. J. 1998. The role of spirochetes in periodontal disease. Adv. Dent. Res. 2:275-283. [DOI] [PubMed] [Google Scholar]
- 32.Miyake, K., H. Ogata, Y. Nagai, S. Akashi, and M. Kimoto. 2000. Innate recognition of lipopolysaccharide by Toll-like receptor 4/MD-2 and RP105/MD-1. J. Endotoxin Res. 6:389-391. [PubMed] [Google Scholar]
- 33.Netea, M. G., M. van Deuren, B. J. Kullberg, J. M. Cavaillon, and J. W. van der Meer. 2002. Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol. 23:135-139. [DOI] [PubMed] [Google Scholar]
- 34.Nixon, C. S., M. J. Steffen, and J. L. Ebersole. 2000. Cytokine responses to Treponema pectinovorum and Treponema denticola in human gingival fibroblasts. Infect. Immun. 68:5284-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogata, H., I. Su, K. Miyake, Y. Nagai, S. Akashi, I. Mecklenbrauker, K. Rajewsky, M. Kimoto, and A. Tarakhovsky. 2000. The Toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J. Exp. Med. 192:23-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogawa, T., Y. Asai, M. Hashimoto, O. Takeuchi, T. Kurita, Y. Yoshikai, K. Miyake, and S. Akira. 2002. Cell activation by Porphyromonas gingivalis lipid A molecule through TLR4- and MyD88-dependent signaling pathway. Int. Immunol. 14:1-8. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa, T., Y. Asai, M. Hashimoto, and H. Uchida. 2002. Bacterial fimbriae activate human peripheral blood monocytes utilizing TLR2, CD14 and CD11a/CD18 as cellular receptors. Eur. J. Immunol. 32:2543-2550. [DOI] [PubMed] [Google Scholar]
- 38.Opitz, B., N. W. Schroder, I. Spreitzer, K. S. Michelsen, C. J. Kirschning, W. Hallatschek, U. Zahringer, T. Hartung, U. B. Gobel, and R. R. Schumann. 2001. Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-kappaB translocation. J. Biol. Chem. 276:22041-22047. [DOI] [PubMed] [Google Scholar]
- 39.Peters, S. R., M. Valdez, G. Riviere, and D. D. Thomas. 1999. Adherence to and penetration through endothelial cells by oral treponemes. Oral Microbiol. Immunol. 14:379-383. [DOI] [PubMed] [Google Scholar]
- 40.Poltorak, A., X. He, I. Smirnova, M.-Y., Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 41.Rams, T. E., M. Andriolo, D. Feik, S. N. Abel, T. M. McGivern, and J. Slots. 1991. Microbiological study of HIV-related periodontitis. J. Periodontol. 62:74-81. [DOI] [PubMed] [Google Scholar]
- 42.Riviere, G. R., K. S. Weisz, L. G. Simonson, and S. A. Lukehart. 1991. Pathogen-related spirochetes identified within gingival tissue from patients with acute necrotizing ulcerative gingivitis. Infect. Immun. 59:2653-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts, F. A., K. A. McCaffery, and S. M. Michalek. 1997. Profile of cytokine mRNA expression in chronic adult periodontitis. J. Dent. Res. 76:1833-1839. [DOI] [PubMed] [Google Scholar]
- 44.Rock, F. L., G. Hardiman, J. C. Timans, R. A. Kastelein, and J. F. Bazan. 1998. A family of human receptors structurally related to Drosophila Toll. Proc. Natl. Acad. Sci. USA 95:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenstein, D. I., G. R. Riviere, and K. S. Elott. 1993. HIV-associated periodontal disease: new oral spirochete found. J. Am. Dent. Assoc. 124:76-80. [DOI] [PubMed] [Google Scholar]
- 46.Ross, K. F., and M. C. Herzberg. 2001. Calprotectin expression by gingival epithelial cells. Infect. Immun. 69:3248-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz, Z., D. D. Dean, and B. D. Boyan. 1996. The biochemistry and physiology of the periodontium, p. 61-107. In T. G. Wilson and K. S. Kornman (ed.), Fundamentals of periodontics. Quintessence Publishing Co., Inc., Chicago, Ill.
- 48.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 50.Takeuchi, O., T. Kawai, H. Sanjo, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, K. Takeda, and S. Akira. 1999. TLR6: a novel member of an expanding Toll-like receptor family. Gene 231:59-65. [DOI] [PubMed] [Google Scholar]
- 51.Takeuchi, O., T. Kawai, P. F. Muhlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 52.Takeuchi, O., S. Sato, T. Horiuchi, K. Hoshino, K. Takeda, Z. Dong, R. L. Modlin, and S. Akira. 2002. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 169:10-14. [DOI] [PubMed] [Google Scholar]
- 53.Tonetti, M. S., M. A. Imboden, L. Gerber, N. P. Lang, J. Laissue, and C. Mueller. 1994. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect. Immun. 62:4005-4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uitto, V. J., Y. M. Pan, W. K. Leung, H. Larjava, R. P. Ellen, B. B. Finlay, and B. C. McBride. 1995. Cytopathic effects of Treponema denticola chymotrypsin-like proteinase on migrating and stratified epithelial cells. Infect. Immun. 63:3401-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Umemoto, T., T. Jinno, Y. Taiji, and T. Ogawa. 2001. Chemotaxis of oral treponemes toward sera and albumin of rabbit. Microbiol. Immunol. 45:571-577. [DOI] [PubMed] [Google Scholar]
- 56.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 57.Vasselon, T., and P. A. Detmers. 2002. Toll receptors: a central element in innate immune responses. Infect. Immun. 70:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson, M., K. Reddi, and B. Henderson. 1996. Cytokine-inducing components of periodontopathogenic bacteria. J. Periodontal Res. 31:393-407. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, J., H. Dong, S. Kashket, and M. J. Duncan. 1999. IL-8 degradation by Porphyromonas gingivalis proteases. Microb. Pathog. 26:275-280. [DOI] [PubMed] [Google Scholar]