Abstract
Complement-mediated opsonization of encapsulated Staphylococcus aureus (CP+) of the predominant capsule types, 5 and 8, remains poorly understood. Our previous work showed that complement is important for mouse survival of CP+ type 5 bacteremia and that the type 5 capsule inhibits the binding of opsonic C3 fragments to the organism. The importance of complement-mediated opsonization of CP+ was tested by neutrophil phagocytosis assays. Complement-mediated opsonization of CP+ increased phagocytosis by 57% compared to opsonization in complement-inhibited serum. Agar-grown CP+, enhancing capsule expression, was phagocytosed only one-tenth as well as the capsule-negative organisms (CP−), supporting the belief that staphylococcal polysaccharide capsules impair phagocytosis. Despite relatively poor phagocytosis of CP+ compared to CP−, complement activation increased the phagocytosis of CP+ by 103%. Thus, complement in normal human serum may have an important role in opsonizing CP+, even when capsule expression is strong. The ability of bound C3 fragments to interact with complement receptor 1 (CD35) on the membrane of human erythrocytes was tested in an immune adherence assay. S. aureus capsule was able to mask C3 fragments on the organism from binding to complement receptor 1. The inhibition of C3 binding to CP+ and the masking of deposited C3 fragments caused by the presence of capsule was associated with markedly decreased phagocytosis. The addition of anti-capsule antibodies to normal human serum was found to markedly improve the recognition of deposited C3 fragments by complement receptor 1 even when the absolute number of C3 molecules bound to S. aureus was not increased.
Staphylococcus aureus is the most common cause of nosocomial bacterial infections and a frequent cause of community-acquired bacterial infections (4, 15). S. aureus accounts for considerable morbidity, mortality, and medical expense and continues to increase in antibiotic resistance (11, 19, 21). Adding immune-directed strategies to antibiotic therapy may provide a means to improve the outcomes of S. aureus infections and decrease medical costs.
The S. aureus serotypes (5 and 8) that account for 85% of clinical isolates are encapsulated (CP+), although the role of capsule in staphylococcal pathogenesis remains unclear (16, 22). Isogenic, capsule-negative mutants (CP−) of serotype 5 and 8 strains are available to evaluate the effects of the polysaccharide capsule (1, 2). Previous investigators have demonstrated that the capsule of these pathogenic strains diminish phagocytosis efficiency and increase mouse lethality from bacteremia (17, 23).
Normal human serum (NHS) from nonimmunized individuals usually contains antibodies that will bind to S. aureus peptidoglycan and may contain antibodies against other S. aureus cell wall components (24, 26). Antibodies that have affinity for the polysaccharide capsule of type 5 and type 8 S. aureus may also be found in NHS but are generally present only at very low levels, i.e., <10 μg/ml (6, 25). A vaccine has recently been developed by using protein-conjugated polysaccharide from the type 5 and 8 strains that promotes the generation of anti-capsule antibody. This vaccine is currently being evaluated for prevention of S. aureus bacteremia in human trials (20).
The complement system serves as an important element of innate immune defense against many bacterial pathogens (reviewed in reference 8). Our previous work demonstrated that mice depleted of complement by cobra venom factor show markedly increased lethality, from 8 to 64%, after bacteremia with CP+ S. aureus (5). We confirmed that minimal capsule is expressed when CP+ organisms are grown to mid-logarithmic phase, but significant capsule is expressed when CP+ organisms are grown on agar or are in stationary-phase growth. When capsule is expressed the binding of opsonic C3 fragments is decreased. We also demonstrated that when CP+ is incubated in serum considerable quantities of both C3b and iC3b, the major complement opsonins, were deposited on both log-phase and stationary-phase organisms.
In the present study, we confirm the opsonic importance of complement and antibody for efficient phagocytosis of CP+ S. aureus. We evaluate the effect of capsule on the availability of C3 fragments bound to the CP+ S. aureus to interact with complement receptors and how this influences phagocytosis. We also investigate how the presence of anti-capsule antibody against CP+ can affect the availability of opsonic C3 fragments to interact with complement receptors.
MATERIALS AND METHODS
Bacterial strains.
The encapsulated S. aureus strain used in these experiments was Reynolds (serotype 5). Strain JL022 is an unencapsulated mutant of strain Reynolds, constructed by allelic replacement mutagenesis, which carries a 727-bp deletion in the cap50 gene (18). The JL022 strain was kindly provided by Jean Lee (Channing Laboratory, Boston, Mass.) Strains were cultivated in Columbia medium with 2% NaCl to enhance capsule expression. Colonies of S. aureus grown overnight were inoculated into Columbia-2% NaCl broth and incubated at 37°C with rotary agitation to the mid-logarithmic phase of growth. Solid-medium-grown organisms were grown on Columbia-2% NaCl agar plates at 37°C for 16 h. It is known that S. aureus Reynolds grown to mid-logarithmic phase, as described above, expresses minimal capsule and, when grown on agar, expresses large amounts of capsule (5).
Complement buffers.
Experiments of complement activation were performed in isotonic Veronal-buffered saline (VBS). In GVBS++ buffer (VBS with 0.1% gelatin, 0.15 mM CaCl2, and 1.0 mM MgCl2), all complement activation pathways are active. In EDTA-GVBS−− buffer (VBS with 0.1% gelatin, 0.01 M EDTA), all complement activation pathways are inhibited.
Complement and immunoglobulin sources.
Healthy volunteers donated NHS that was shown to have normal levels of total hemolytic complement and total alternative hemolytic pathway complement activity. Blood was obtained for serum in Vacutainer (Becton Dickinson, Franklin Lakes, N.J.) tubes without additives, allowed to clot at room temperature, and centrifuged to remove cellular components. Serum samples were stored at −80°C for up to 3 months.
Antistaphylococcal-antibody-depleted serum samples were prepared by adsorbing 1 ml of NHS with 1010 CFU of solid-grown Reynolds organisms at 0°C (to prevent complement activation) for 1 h. This procedure was repeated for a total of three adsorptions for each sample.
Anti-capsule antibody (Altastaph, Nabi, Inc., Rockville, Md.) was kindly provided by A. Fattom. Altastaph is a human polyclonal product derived from the pooled sera of individuals immunized with conjugated capsule polysaccharide 5 and capsule polysaccharide 8 (6).
Neutrophils and erythrocytes.
Blood for neutrophils was obtained from healthy human volunteers in Vacutainer K3EDTA tubes. The neutrophil layer was recovered after sedimentation with neutrophil isolation media NIM (Cardinal Associates, Inc., Santa Fe, N.Mex.). Neutrophils were washed twice with Hanks balanced salt solution (HBSS; Gibco, Grand Island, N.Y.), incubated for 10 s in distilled H2O to lyse the remaining erythrocytes, and then returned to HBSS. This cell suspension was then checked by trypan blue staining for cell viability (>95%), and cell density was determined by using a hemocytometer. Cell density was then brought to 5 × 105 cells/ml.
Erythrocytes for immune adherence experiments were obtained from healthy volunteers in Vacutainer K3EDTA tubes. Cells were washed in EDTA-GVBS−− buffer twice and incubated in the same buffer for 20 min at 37°C. Cells were then washed twice in GVBS++ buffer and brought to 0.5 × 108 cells/ml by photospectroscopy.
S. aureus opsonization.
Bacteria grown to mid-logarithmic phase in liquid media were washed twice in GVBS++ buffer and suspended in the same buffer to a concentration of 109 CFU/ml by photospectroscopy at 600 nm. Bacteria grown on agar were suspended in GVBS++ buffer and diluted to 109 CFU/ml. Bacteria were then diluted to 108 CFU/ml in GVBS++ buffer and incubated with 2% serum, unless otherwise noted, at 37°C with agitation for 30 min. This concentration of serum was shown to provide maximal C3 binding in earlier studies (5). The bacteria were then washed twice with cold EDTA-GVBS−− buffer and suspended to 108 CFU/ml in GVBS++ buffer. No decrease in S. aureus viability was found in any of the complement buffers used over 30 min.
Phagocytosis assays.
Opsonized bacteria were washed and suspended to 108 CFU/ml. Neutrophil suspensions (5 × 105 cells/ml) and bacterial suspensions were mixed in equal volumes on a coverslip and incubated at 37°C in 5% CO2 for 30 min. The coverslips were washed with HBSS four times, stained with 0.01% acridine orange (Sigma-Aldrich, St. Louis, Mo.) for 70 s, washed in HBSS, and counterstained with 0.05% crystal violet in 0.15 M NaCl to quench acridine orange staining of extracellular bacteria. The coverslips were then washed in HBSS, washed in distilled H2O, dried, and mounted. Bacteria and neutrophils were visualized by fluorescent microscopy by using acridine orange-specific filters. Each coverslip was visualized under oil immersion with a ×100 magnification lens by a third party who played no role in the experimental design or analysis. Three to five randomly selected high-power fields were visualized for each slide, and the number of neutrophils and number of bacteria engulfed in neutrophils were summed and averaged as the number of bacteria/neutrophil.
Immune adherence assays.
The immune adherence test evaluates the ability of membrane-bound human CR1, present on the surface of human erythrocytes, to interact with particle bound C3 causing cross-agglutination (3, 10). Opsonized bacterial suspensions were washed and added to human erythrocytes to achieve a total volume of 60 μl containing 2.5 × 106 erythrocytes and 2.5 × 106 bacteria. These mixtures were then placed in wells of a 96-well U-bottom plate (Nalge Nunc International, Naperville, Ill.) and incubated for 1 h at room temperature prior to reading. Readings were graded visually for aggregation on a scale of 0 to 4+, compared to positive and negative immune adherence controls.
C3 binding quantitation by 125I-labeled anti-C3 antibody.
Goat anti-human C3 antibody that recognizes all C3 fragments was prepared in our laboratory. The immunoglobulin G fraction was purified by caprilic acid and ammonium sulfate precipitation. The antibodies were radiolabeled with 125I (Amersham Co., Arlington Heights, Ill.) by using Iodobeads (Pierce Chemical Co., Rockford, Ill.) to a specific activity of 3.3 × 105 cpm/μg. Radiolabeled anti-C3 antibody was added to unlabeled anti-C3 antibody at a 1:50 dilution.
To determine the relative amount of C3 bound, opsonized and washed S. aureus organisms at a concentration of 5 × 107 CFU/ml were incubated in a saturating quantity of trace-radiolabeled anti-C3 antibody (0.025 mg/ml) for 60 min at room temperature. The bacteria were thoroughly washed, and the counts per minute (cpm) were determined by gamma spectrometer (Packard Cobra II, Meridien, Conn.). Specific binding was determined by subtracting the cpm/106 CFU in EDTA-GVBS−− buffer from the total cpm/106 CFU in GVBS++ buffer.
Determination of C3 binding by 125I-C3.
As an independent quantitative C3 standard, purified human C3 (Advanced Research Technologies, Inc., San Diego, Cal.) was trace radiolabeled with 125I by using Iodobeads to a specific activity of 106 cpm/μg. 125I-labeled C3 (125I-C3) was added to serum at a 1:50 ratio of labeled C3 to unlabeled C3 molecules.
Opsonized and washed S. aureus at 5 × 107 CFU/ml were incubated with 2% serum containing trace amounts of 125I-C3 in standard complement buffers for 30 min at 37°C. The bacteria were thoroughly washed, and the cpm were determined by using a gamma spectrometer. The total numbers of C3 molecules bound per bacterium were calculated as follows: [(cpm/cpm per molecule of 125I-C3) × (50 molecules of unlabeled C3/molecule of 125I-C3)]/CFU in the pellet. Specific binding was determined by subtracting C3/CFU in EDTA-GVBS−− buffer from C3/CFU in GVBS++ buffer.
C3 fragments on agar-grown S. aureus.
Agar-grown CP+ organisms were opsonized in fresh human serum either with 1% anti-capsule antibody or without anti-capsule antibody. EA were prepared to provide a standard for iC3b fragments by sensitizing sheep erythrocytes with anti-Forssman antigen antibody (i.e., EA) and incubation in 10% C8-depleted human serum at 37°C for 30 min. iC3b-coated EA were then lysed with water to remove hemoglobin. Opsonized bacteria and lysed EA were washed and then incubated with 25 mM methylamine for 60 min at 37°C to release ester linkage-bound C3 fragments attached to the organism surface. The bacteria were pelleted by centifugation, and the supernatants were recovered and processed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blot analysis was performed by using goat anti-human C3 antibody (generated in our laboratory) and enhanced chemiluminescence.
Statistical analysis.
Error bars indicate the standard deviation (Excel 98; Microsoft Co., Redmond, Wash.) or the standard error of the sample means (VassarStats [www.faculty.vassar.edu/∼lowry/VassarStats.html]), as indicated. P values were determined by using the unpaired two-tailed Student t test assuming unequal variance (Microsoft Excel 98).
RESULTS
Complement and antibody contribute to the phagocytosis of pathogenic S. aureus.
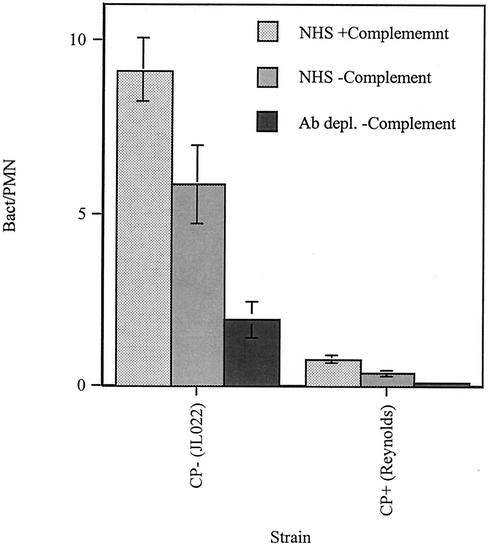
Phagocytosis assays were first performed to test the relative contributions of complement, antibody present in the serum of nonimmunized individuals, and staphylococcal polysaccharide capsule on neutrophil phagocytosis efficiency (Fig. 1). Phagocytosis of agar-grown CP− bacteria opsonized by complement in NHS was 57% more extensive (bacteria/neutrophil) compared to phagocytosis in EDTA-serum, in which complement activation was prevented (P = 0.03). When grown on agar, conditions that enhance capsule expression of encapsulated organisms, the unencapsulated isogenic mutant (CP−) was phagocytosed 10 times more efficiently than the encapsulated strain (CP+). Interestingly, although agar-grown CP+ was poorly phagocytosed compared to the CP− strain, complement-mediated opsonization of CP+ in NHS increased phagocytosis 103% compared to opsonization in complement-inactive EDTA-serum (P = 0.006). Under conditions that did not allow complement to activate, the presence of antibodies in NHS improved phagocytosis by 200% for the CP− strain compared to sera in which antibodies were removed by adsorption against S. aureus (P = 0.007). For the CP+ strain, under the same conditions preventing complement activation, the antibodies present in NHS increased phagocytosis 225% compared to antibody-depleted adsorbed serum (P = 0.01).
FIG. 1.
Neutrophil phagocytosis efficiency for CP+ and CP− strains grown on agar and opsonized in 2% serum with complement active in GVBS++ buffer (NHS + complement), in 2% serum with complement activation inhibited in EDTA-GVBS−− buffer (NHS − complement), and in 2% anti-staphylococcal antibody-depleted serum with complement activation inhibited (Ab depl. − Complement). Phagocytosis efficiency was determined by acridine orange-crystal violet staining of neutrophils and counting the engulfed bacteria by using fluorescence microscopy. Each datum point represents the mean number of bacteria/neutrophil for four to five high-power fields from four independent experiments; more than 100 neutrophils were counted for each experiement. The error bars represent the standard error of the mean (SEM).
Phagocytosis was also tested for the conditions of opsonization with 2% antibody depleted serum without complement inhibition. We found bacterium-per-polymorphonuclear-leukocyte values to be similar to those for the condition of antibody depletion plus complement inhibition (data not shown). This finding is consistent with our previous observation that C3 binding to S. aureus in 2% serum is 90% by the classical pathway and suggests that for efficient phagocytosis of S. aureus in 2% serum the classical pathway is more critical than the alternative pathway.
Phagocytosis efficiency of encapsulated S. aureus with increasing concentrations of serum.
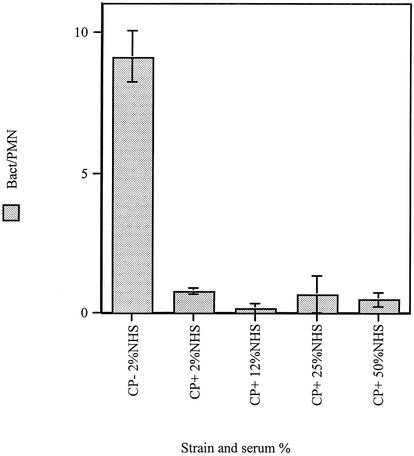
We were interested in whether the antiphagocytic effects of the polysaccharide could be overcome by incubating the bacteria in increasing concentrations of serum providing more opsonins to deposit on the CP+ S. aureus surface. For the solid-grown CP+ strain, increasing serum concentrations from 2% even up to 50% did not show any appreciable improvement in phagocytosis efficiency (Fig. 2).
FIG. 2.
Neutrophil phagocytosis efficiency for CP+ and CP− strains grown on agar and opsonized in various concentrations of NHS. Phagocytosis efficiency was determined by acridine orange-crystal violet staining of neutrophils and by counting the engulfed bacteria by using fluorescence microscopy. Each datum point represents the mean number of bacteria/neutrophil for three to five high-power fields for three independent experiments. The error bars represent the SEM.
Immune adherence with encapsulated and unencapsulated S. aureus.
As one way of evaluating the availability of opsonic C3 fragments on the S. aureus surface to interact with cellular membrane bound complement receptors, we studied immune adherence (Table 1). If the complement receptor for C3b (CD35) on human erythrocytes is able to bind its ligand on the surface of C3-coated bacteria, the erythrocytes demonstrate a pattern of agglutination termed positive immune adherence. The quantity of C3 bound to the bacteria was also examined to evaluate the importance of total C3 on the bacteria relative to the availability of the ligand to bind its receptor. The CP+ strain Reynolds was tested in mid-logarithmic phase, when little capsule is expressed, and after growth on solid media, when considerable capsule is expressed. In mid-logarithmic phase the degree of immune adherence correlates positively with the amount of C3 deposited on the S. aureus surface. When grown on agar the CP+ strain is always immune adherence negative.
TABLE 1.
Immune adherence and C3 binding for CP+ strain grown to mid-logarithmic phase or grown on agar
| Serum concn (%) | Agar-grown CP+
|
Mid-log-phase CP+
|
||
|---|---|---|---|---|
| Immune adherence ± SD | C3 bound ± SD (cpm/106 CFU) | Immune adherence | C3 bound ± SD (cpm/106 CFU) | |
| 2 | 0 | 2.2 ± 0.1 | 4+ | 4.2 ± 1.1 |
| 1 | 0 | 0.8 ± 0.3 | 3+ | 1.4 ± 0.4 |
| 0.5 | 0 | 0.2 ± 0.1 | 2+ | 0.7 ± 0.5 |
C3 binding, immune adherence, and phagocytosis for CP+ and CP− S. aureus.
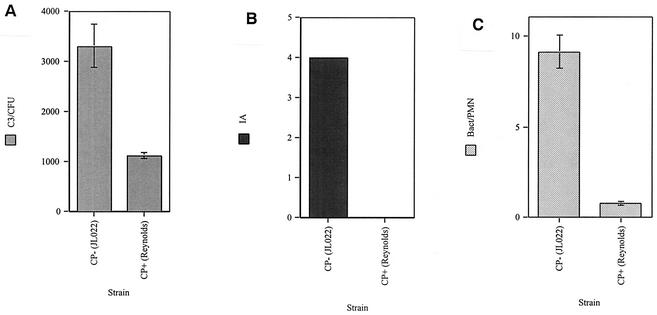
The binding of the major complement opsonin C3 and the availability of bound C3 fragments to interact with erythrocyte complement receptors, as indicated by immune adherence, were correlated with their relationship to phagocytosis by neutrophils (Fig. 3). These tests were performed with agar-grown encapsulated S. aureus and its isogenic capsule-negative mutant. C3 binding was three times greater for the CP− strain than for the CP+ strain (P = 0.003). Immune adherence for the CP− strain was maximal, whereas immune adherence for the CP+ strain was undetectable. Phagocytosis by neutrophils was 10 times greater for the CP− strain than the CP+ strain (P < 0.001).
FIG. 3.
Agar-grown CP+ and CP− strains opsonized in 2% NHS and evaluated for C3 binding (C3/CFU) (A), immune adherence (AI) (B), and phagocytosis by neutrophils (C3/PMN) (C). C3 binding was determined by adding trace radiolabeled C3 to NHS and counting the gamma emissions. Each datum point represents the mean of six separate determinations. Immune adherence was determined by incubation of opsonized bacteria with human erythrocytes followed by visual scoring. The readings were identical for four separate determinations. Phagocytosis efficiency was determined by acridine orange-crystal violet staining of neutrophils and counting engulfed bacteria by fluorescence microscopy. Each datum point represents the mean number of bacteria/neutrophil for four to five high-power fields from four independent experiments. The error bars represent the SEM.
Immune adherence and C3 binding in the presence of anti-capsule antibody.
In order to evaluate the effect of anti-capsule antibody on the availability of bound C3 fragments to interact with complement receptors immune adherence was tested with agar-grown CP+ that we have shown have high capsule expression (Table 2). C3 binding to the organism was also assessed to determine whether effects produced by anti-capsule antibody are attributable to the amount of C3 on the S. aureus surface. As previously demonstrated immune adherence with agar-grown CP+ is undetectable at all serum concentrations. Interestingly, the amount of C3 binding was equal for anti-capsule antibody concentrations of 1, 0.2, and 0%. However, immune adherence increased in a dose-response-related manner as anti-capsule antibody was added to the serum. The anti-capsule antibody did not cause immune adherence in the absence of complement.
TABLE 2.
Immune adherence and C3 binding for CP+ strain grown on agar with anti-capsule antibody added to 2% NHS
| Antibody (concn [%]) | CP+ Strain
|
|
|---|---|---|
| Immune adherence ± SD | C3 bound ± SD (cpm/106 CFU) | |
| Anti-CP+ (1) | 3+ | 5.8 ± 0.4 |
| Anti-CP+ (0.2) | 1+ | 6.2 ± 0.2 |
| Anti-CP+ (0) | 0 | 5.9 ± 0.2 |
C3 fragments on agar-grown CP+ after incubation with serum and anti-capsule antibody.
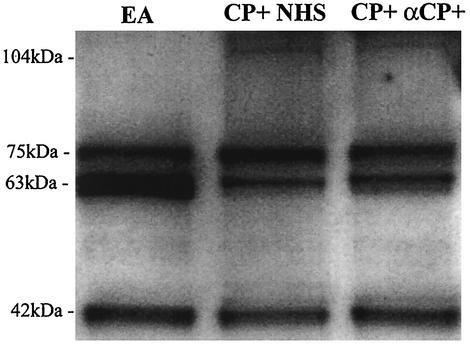
In order to evaluate whether anti-capsule antibody changes the fragments of C3 found on the S. aureus surface, we examined bacterial eluates by Western blot analysis (Fig. 4). This analysis of C3 fragments on agar-grown CP+ after incubation with fresh human serum showed predominantly iC3b fragments, as well as C3b fragments. The addition of 1% anti-capsule antibody, a sufficient quantity to dramatically increase immune adherence, to the serum prior to incubation with agar-grown CP+ showed a C3 fragment pattern identical to that seen in the absence of anti-capsule antibody.
FIG. 4.
Deposited C3 fragments on EA (antibody-sensitized sheep erythrocytes), agar-grown CP+ strain Reynolds incubated with 10% NHS (CP+ NHS), and agar-grown CP+ incubated with 10% NHS with 1% anti-capsule antibody (CP+ αCP+). The bound C3 fragments were released by treatment with 25 mM methylamine and then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting with anti-C3 antibodies. The 104-kDa band represents the C3b α′ chain, the 75-kDa band represents the C3 β chain, the 63-kDa band represents iC3b α′1 chain, and the 42-kDa band represents the iC3b α′2 chain. The C3 fragments bound to EA are all from iC3b; both the C3b and the iC3b forms are found bound to strain Reynolds.
DISCUSSION
Complement is a critical component of innate immunity and in vitro experiments suggest that complement is important in increasing neutrophil phagocytosis of several staphylococcal strains (13, 27). We have previously reported that complement is important for survival of CP+ S. aureus bacteremia in mouse models and that the presence of capsule decreases binding of opsonic complement C3 fragments (5). However, the activation of complement, the availability of bound opsonic complement to interact with membrane complement receptors and the effect of anti-capsule antibody on C3 binding have not been evaluated for their influence on the phagocytosis of pathogenic type 5 encapsulated S. aureus.
These experiments show that complement activation and the antibodies in serum, from individuals not specifically immunized, strongly improves phagocytosis. We confirm, as other investigators have demonstrated, that strong capsule expression by CP+ strains decreases phagocytosis compared to CP− isogenic strains under the same conditions (17, 23). For the CP− strain it was expected that complement activation and the presence of antibodies in NHS would strongly increase phagocytosis as these opsonins should be readily available for interaction with their receptors on the neutrophil surface. One might expect that the effects of opsonins on phagocytosis of the CP+ strain grown on agar, promoting strong capsule expression, would be partially masked by the capsule. As expected, phagocytosis efficiency of agar-grown CP+ organisms was only one-tenth that of CP− organisms. Surprisingly, complement activation still increased phagocytosis for the CP+ strain by 103%, even though the overall efficiency of phagocytosis of CP+ was much less than for CP−. This suggests that the capsule is only partially protective against opsonins and complement activation is still important for controlling CP+ S. aureus, even under conditions of strong capsule expression. This is consistent with our previous findings that circulating complement is important for mouse survival of injection with agar-grown CP+ S. aureus (5).
Initial experiments showed that capsule strongly inhibited phagocytosis of CP+ at a low serum concentration of 2%. We felt it would be important to know whether this inhibitory effect on phagocytosis could be overcome at higher serum concentrations, i.e., concentrations closer to that which might be encountered in the blood. Compared to 2% serum even serum concentrations of 50% caused no discernible increase in phagocytosis for CP+. We have previously shown that C3 binding to CP+ at 30 min plateaus at 2 to 10% serum with all complement pathways active (5). This suggests that lack of improved phagocytosis after CP+ incubation at higher serum concentrations may reflect, at least in part, the saturation of C3 binding sites and the fact that increasing serum concentration does not overcome the effect of capsule.
Tests of immune adherence examine the ability of complement receptors on the cell membrane of human erythrocytes to interact with and bind to opsonic C3 fragments. For CP+ grown to mid-logarithmic phase, when little capsule is expressed, immune adherence increased after incubation in increasing concentrations of serum and correlated nicely with the amount of C3 fragments bound to the organism. When CP+ was grown on agar to promote capsule expression immune adherence was always negative, even when sufficient amounts of C3 were deposited to cause very strong immune adherence for the mid-logarithmic-phase organism. This suggests that the capsule of pathogenic S. aureus not only partially inhibits C3 binding but also masks deposited C3 fragments making them less available to complement receptors for C3. The fact that complement activation can increase phagocytosis efficiency when capsule is strongly expressed and immune adherence is negative suggests that CR1 on erythrocytes are less sensitive in detecting bacterium-bound C3 fragments than are neutrophils, which have CR1 and CR3 receptors and a more flexible cell membrane.
C3 binding and immune adherence were also tested with agar-grown CP+ and CP− organisms and compared to phagocytosis efficiency by neutrophils. S. aureus capsule decreased the binding of C3 fragments to the organism and strongly inhibited immune adherence, again suggesting that capsule diminished the ability of complement receptors on erythrocytes to interact with C3 fragments on the bacteria. The findings correlated with the strong inhibition of phagocytosis of CP+ organisms compared to CP− S. aureus. This suggests that the decreased phagocytosis caused by S. aureus capsule may, at least in part, result from fewer complement receptors on neutrophils being able to bind to C3 fragments deposited on the S. aureus surface.
Previous investigators have shown that anti-capsule antibody can increase phagocytosis efficiency of CP+ S. aureus and decrease lethality from bacteremia in mice and decrease endocarditis in rats (7, 14). It has also been shown that serum and anti-capsule polyclonal or serum and monoclonal antibody increases phagocytosis efficiency of CP+ S. aureus compared to nonimmune serum (13). These findings suggest that the CP+ organisms are better opsonized in the presence of serum and anti-capsule antibody compared to the serum of nonimmunized donors. We speculated that some of the improved opsonization may be related to the increased availability of deposited complement fragments. Adding anti-capsule antibody to serum before serum opsonization of CP+ resulted in strongly positive immune adherence compared with no immune adherence for complement bound in the absence of anti-capsule antibody. Surprisingly, under the conditions of the present study the total numbers of C3 bound to the organisms did not change with the addition of anti-capsule antibody. This suggests that anti-capsule antibody increases the accessibility of deposited C3 fragments to interact with complement receptors. Presumably, this occurs by causing more C3 fragments to bind directly to the capsule or to antibody on the capsule surface instead of to the cell wall, thus in a more favorable location for recognition by complement receptors.
We speculated that the increase in immune adherence for agar-grown CP+ incubated with serum with 1% anti-capsule antibody could result from a change in the C3 fragments deposited on the S. aureus surface or capsule. Complement receptor 1 (CD35) is believed to have a greater affinity for C3b than iC3b, and C3b bound to antibody is relatively resistant to conversion to iC3b by the action of factors H and I (9). Western blot analysis of the C3 fragments deposited on the agar-grown CP+ did not show a change in the relative quantities of C3b and iC3b on the S. aureus surface with the addition of anti-capsule antibody to serum. The numbers of C3 fragments on the S. aureus surface and the ratio of C3b and iC3b fragments on the S. aureus surface were not changed by anti-capsule antibody. This further suggests that the increase in immune adherence caused by anti-capsule antibody results from increased availability of C3 fragments to interact with membrane complement receptors.
Our findings suggest that complement-mediated opsonization of encapsulated type 5 S. aureus remains critical for efficient neutrophil phagocytosis, even though the capsule prevents optimal complement opsonization. Anti-capsule antibody dramatically improves the phagocytosis efficiency of complement-mediated opsonophagocytosis of S. aureus in vitro. This suggests that if anti-capsule S. aureus antibody were used therapeutically, complement-mediated opsonophagocytosis of S. aureus might be improved, presumably producing better host defense against this organism. Our findings parallel those found for complement-mediated opsonophagocytosis of encapsulated S. pneumoniae and E. coli. For encapsulated S. pneumoniae complement activity and anti-capsule antibody have been shown to greatly improve phagocytosis efficiency by neutrophils and improve guinea pig survival of bacteremia (3). Encapsulated E. coli also require complement activity and bacterium-specific antibody to optimize phagocytosis by neutrophils (12).
In summary, we show in this report that complement activation and antibodies in nonimmune serum are important for efficient phagocytosis of pathogenic encapsulated S. aureus. The expression of capsule by CP+ organisms decreases the ability of cell-bound complement receptors to interact with C3 fragments deposited on the S. aureus. The decreased binding of C3 fragments to CP+ and masking of bound C3 fragments by capsule is associated with markedly decreased phagocytosis by neutrophils. The presence of anti-capsule antibodies during complement activation markedly improves the availability of C3 fragments bound to CP+ to interact with complement receptors.
Acknowledgments
This work was supported by Public Health Service grant AI-01835 for the National Institute of Allergy and Infectious Diseases.
We thank Ali Fattom of Nabi, Inc., for kindly providing the anti-capsule antibody, Altastaph, and Jean Lee of the Channing Laboratory for kindly providing the capsule-negative S. aureus strain JL022.
Editor: J. D. Clements
REFERENCES
- 1.Albus, A., R. D. Arbeit, and J. C. Lee. 1991. Virulence of Staphylococcus aureus mutants altered in type 5 capsule expression. Infect. Immun. 59:1008-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baddour, L. M., C. Lowrance, A. Albus, J. H. Lowrance, S. K. Anderson, and J. C. Lee. 1992. Staphylococcus aureus microcapsule expression attenuates bacterial virulence in a rat model of experimental endocarditis. J. Infect. Dis. 165:749-753. [DOI] [PubMed] [Google Scholar]
- 3.Brown, E. J., S. W. Hosea, C. H. Hammer, C. G. Burch, and M. M. Frank. 1982. A quantitative analysis of the interactions of antipneumococcal antibody and complement in experimental pneumococcal bacteremia. J. Clin. Investig. 69:85-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1996. National Nosocomial Infection Surveillance System report: data summary from October 1986 to April 1996. U.S. Department of Health and Human Services, Atlanta, Ga.
- 5.Cunnion, K. M., J. C. Lee, and M. M. Frank. 2001. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect. Immun. 69:6796-6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fattom, A., R. Schneerson, D. C. Watson, W. W. Karakawa, D. Fitzgerald, I. Pastan, X. Li, J. Shiloach, D. A. Bryla, and J. B. Robbins. 1993. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsule polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect. Immun. 61:1023-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fattom, A. I., J. Sarwar, A. Oritz, and R. Naso. 1996. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect. Immun. 64:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank, M. M. 1995. The complement system, p. 331-353. In M. M. Frank (ed.), Samter's immunologic diseases. Little, Brown, & Co., Boston, Mass.
- 9.Fries, L. F., T. A. Gaither, C. H. Hammer, and M. M. Frank. 1984. C3b covalently bound to IgG demonstrates a reduced rate of inactivation by factors H and I. J. Exp. Med. 160:1640-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaither, T. A., C. H. Hammer, and M. M. Frank. 1979. Studies of the molecular mechanisms of C3b inactivation and a simplified assay of β1H and the C3b inactivator (C3bINA). J. Immunol. 123:1195-1204. [PubMed] [Google Scholar]
- 11.Goetz, A., K. Posey, J. Flemming, S. Jacobs, L. Boody, M. M. Wagener, and R. R. Muder. 1999. Methicillin-resistant Staphylococcus aureus in the community: a hospital-based study. Infect. Control Hosp. Epidemiol. 20:689-691. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz, M. A., and S. C. Silverstein. 1980. Influence of Escherichia coli capsule on the complement fixation and on phagocytosis and killing by human phagocytes. J. Clin. Investig. 65:82-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karakawa, W. W., A. Sutton, R. Schneerson, A. Karpas, and W. F. Vann. 1988. Capsular antibodies induce type-specific phagocytosis of capsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect. Immun. 56:1090-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, J. C., J.-S. Park, S. E. Shepherd, V. Carey, and A. Fattom. 1997. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect. Immun. 65:4146-4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 16.Na'was, T., A. Hawwari, E. Hendrix, J. Hebden, R. Edelman, M. Martin, W. Campbell, R. Naso, and A. I. Fattom. 1998. Phenotypic and genotypic characterization of nosocomial Staphylococcus aureus isolates from trauma patients. J. Clin. Microbiol. 36:414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson, I. M., J. C. Lee, T. Bremell, C. Ryden, and A. Tarkowski. 1997. The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis. Infect. Immun. 65:4216-4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Portoles, M., K. B. Kiser, N. Bhasin, K. H. N. Chan, and L. C. Lee. 2001. Staphylococcus aureus Cap5O has UDP-ManNAc dehydrogenase activity and is essential for capsule expression. Infect. Immun. 69:917-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin, R. J., C. A. Harrington, A. Poon, K. Dietrich, J. A. Greene, and A. Moiduddin. 1999. The economic impact of Staphylococcus aureus infection in New York City hospitals. Emerg. Infect. Dis. 5:9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinefield, H., S. Black, A. Fattom, G. Horwith, S. Rasgon, J. Ordonez, H. Yeoh, D. Law, J. B. Robbins, R. Schneerson, L. Muenz, S. Fuller, J. Johnson, B. Fireman, H. Alcorn, and R. Naso. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346:491-496. [DOI] [PubMed] [Google Scholar]
- 21.Smith, T. L., M. L. Pearson, K. R. Wilcox, C. Cruz, M. V. Lancaster, B. Robinson-Dunn, F. C. Tenover, M. J. Zervos, J. D. Band, E. White, and R. W. Jarvis. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 22.Sompolinsky, D., Z. Samra, W. W. Karakawa, W. F. Vann, R. Schneerson, and Z. Malik. 1985. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J. Clin. Microbiol. 22:828-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thakker, M., J. S. Park, V. Carey, and J. C. Lee. 1998. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect. Immun. 66:5183-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verbrugh, H. A., R. Peters, M. Rozenberg-Arska, P. K. Peterson, and J. Verhoef. 1981. Antibodies to cell wall peptidoglycan of Staphylococcus aureus in patients with serious staphylococcal infections. J. Infect. Dis. 144:1-9. [DOI] [PubMed] [Google Scholar]
- 25.Welch, P. G., A. Fattom, J. Moore, R. Schneerson, J. Shiloach, D. A. Bryla, X. Li, and J. B. Robbins. 1995. Safety and immunogenicity of Staphylococcus aureus type 5 capsular polysaccharide-Pseudomonas aeruginosa recombinant exoprotein A conjugate vaccine in patients on hemodialysis. J. Am. Soc. Nephrol. 7:247-253. [DOI] [PubMed] [Google Scholar]
- 26.Wergeland, A. I., L. R. Haaheim, O. B. Natas, F. Wesenberg, and P. Oeding. 1989. Antibodies to staphylococcal peptidoglycan and its peptide epitopes, teichoic acid, and lipoteichoic acid in sera from blood donors and patients with staphylococcal infections. J. Clin. Microbiol. 27:1286-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu, S., R. D. Arbeit, and J. C. Lee. 1992. Phagocytic killing of encapsulated and microencapsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect. Immun. 60:1358-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]