Abstract
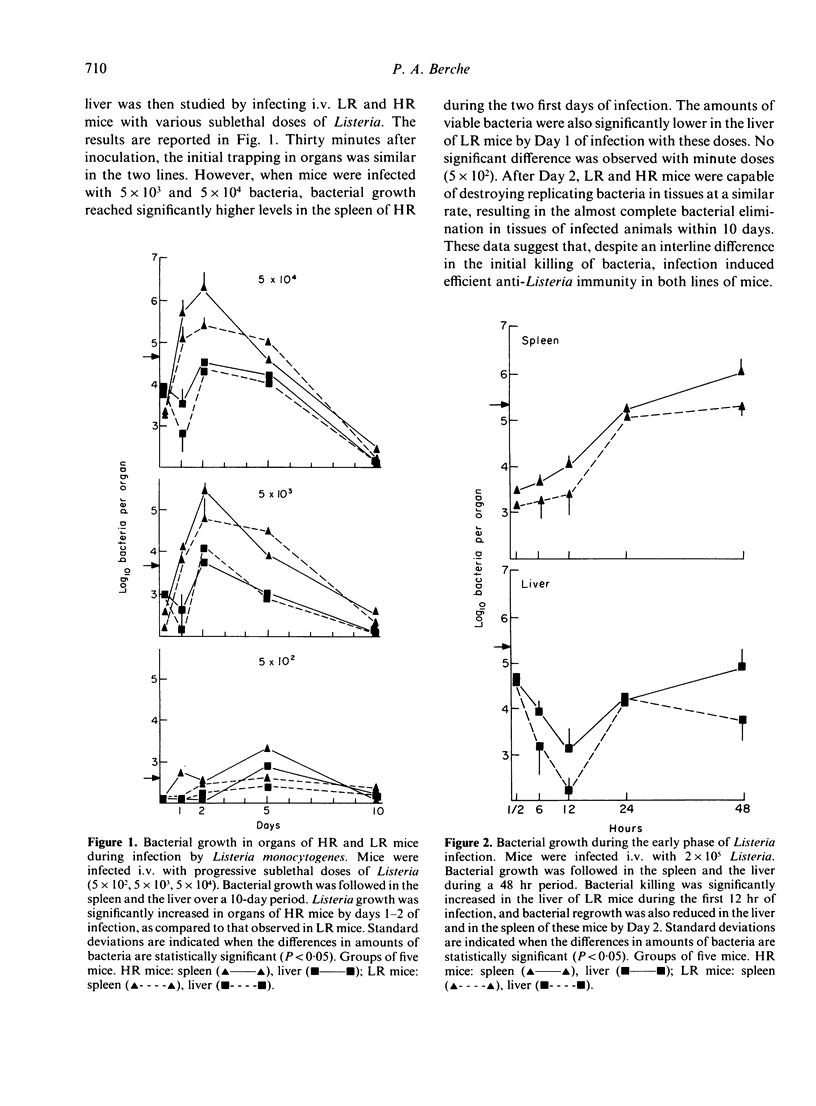
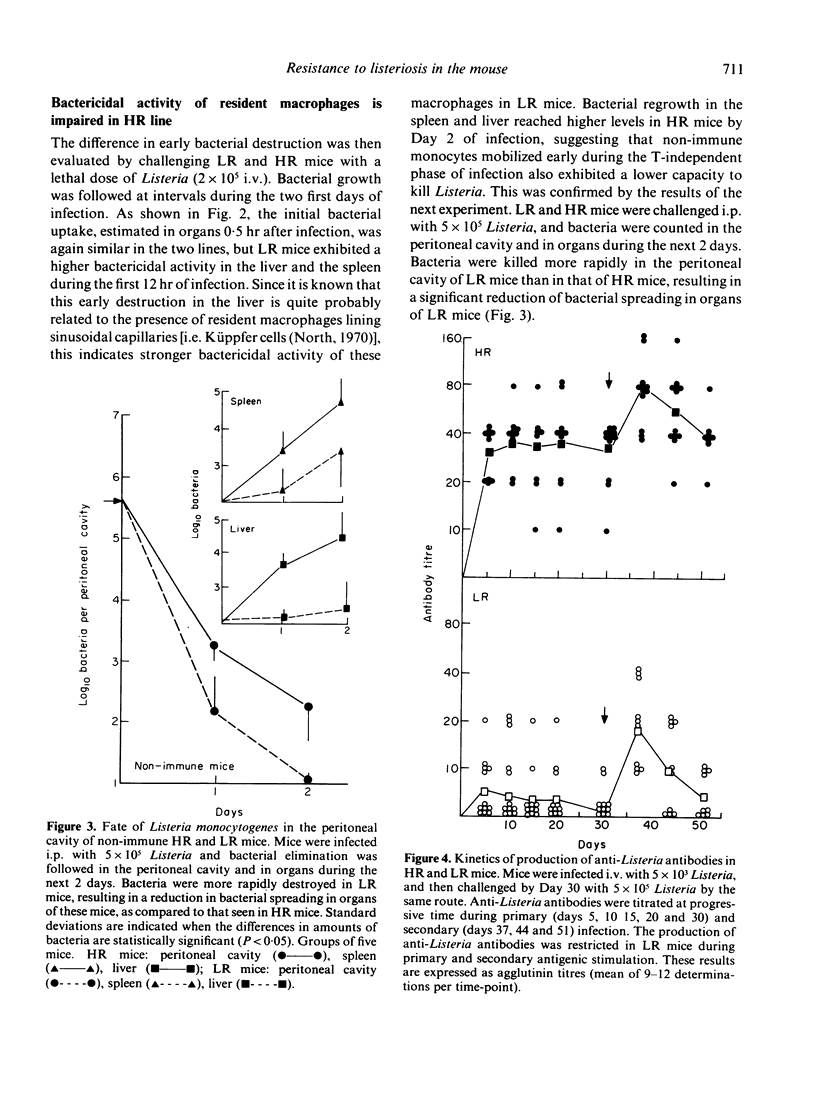
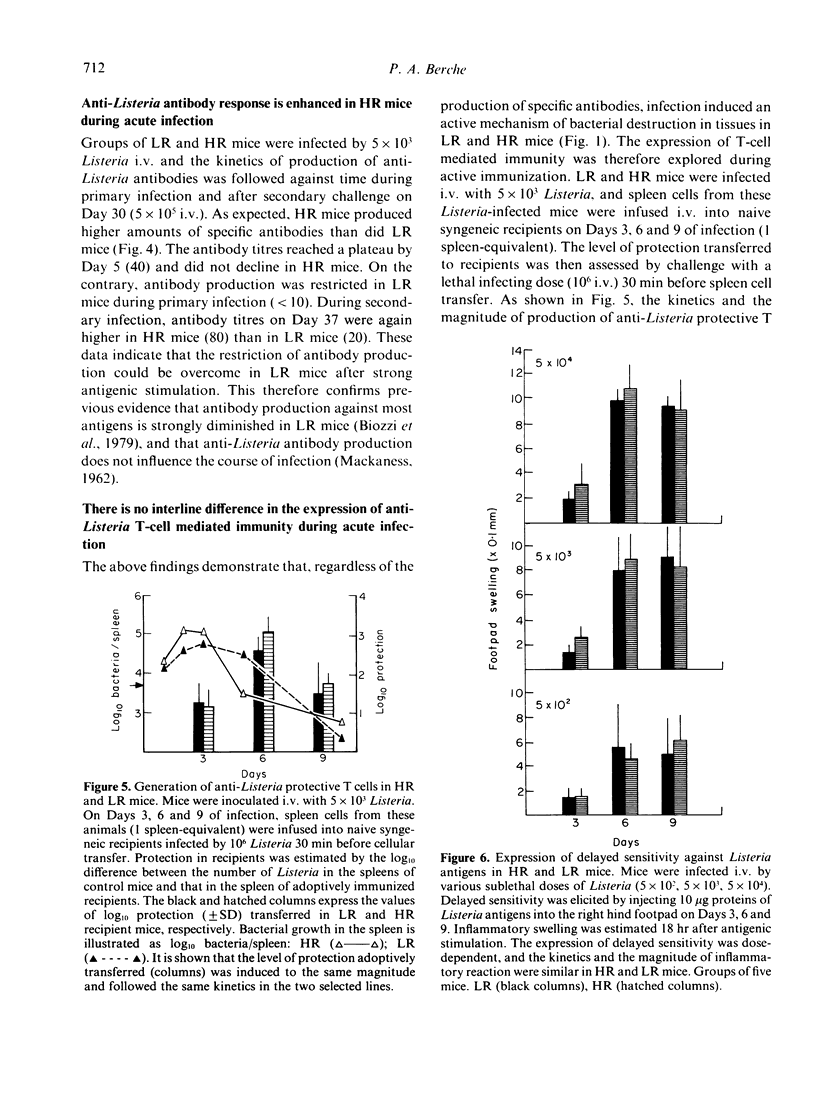
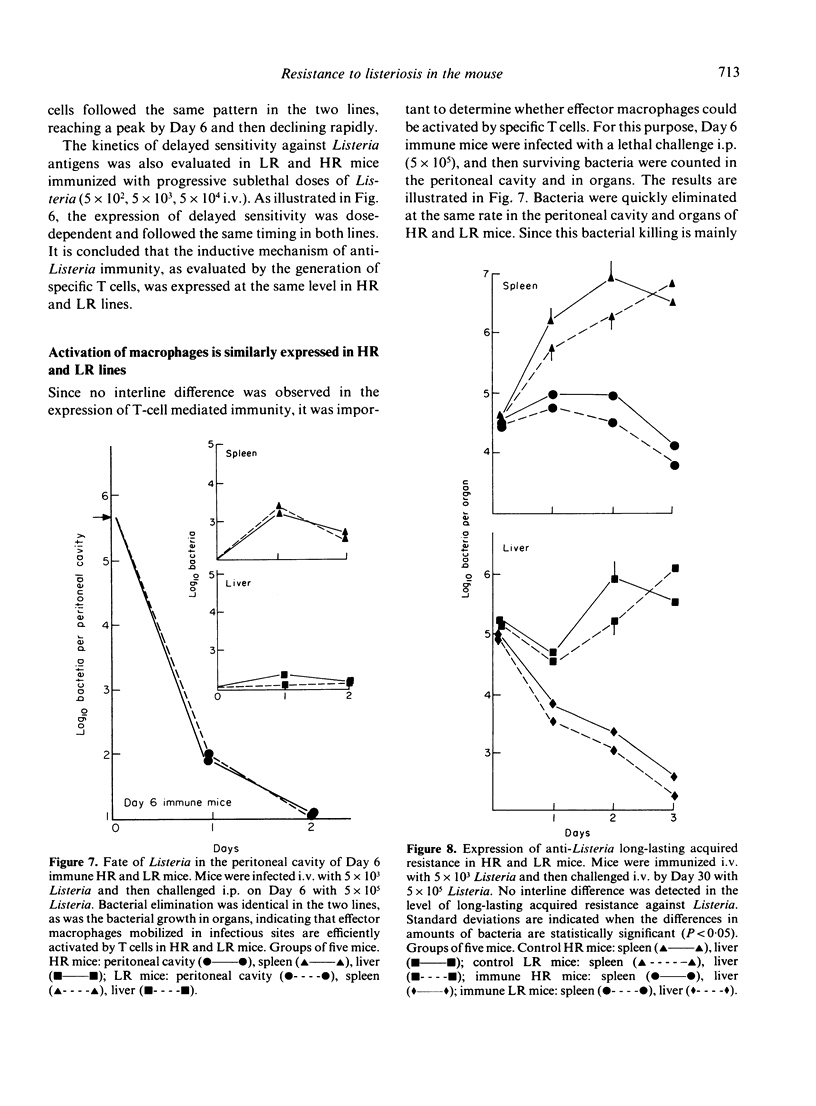
Infection by the intracellular parasite Listeria monocytogenes was studied in two inbred lines of mice genetically selected for high and low antibody production against xenogeneic red blood cells. It was revealed that, during the early non-specific phase of infection, bacterial growth in tissues was significantly enhanced in high responder (HR) mice, as opposed to low responder (LR) mice. This is interpreted as the in vivo expression of a genetic impairment of the bactericidal activity of resident macrophages in this line of mice. After Day 2 of infection, the kinetics of bacterial growth in the spleen and the liver was almost identical in the two lines, indicating that mice from both lines generated efficient anti-Listeria immunity. This was confirmed by the fact that no interline difference could be detected in the expression of T-cell mediated immunity, as estimated by the production of protective T cells and delayed sensitivity T cells, and by the level of immunological memory. The genetic impairment in the bactericidal activity of resident macrophages resulted in a significant increase of anti-Listeria antibody production in HR mice and did not prevent T-dependent activation of effector macrophages mobilized in infectious sites. This explains that the overall resistance to listeriosis was similar in LR and HR mice, as shown by the LD50 values respectively estimated as 2.2 X 10(5) and 3.8 X 10(5) bacteria per mouse. This natural resistance was expressed at the same level as that of C57BL/6 mice.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Doria G. Defective antigen presentation by macrophages from mice genetically selected for low antibody response. Eur J Immunol. 1981 Dec;11(12):984–989. doi: 10.1002/eji.1830111207. [DOI] [PubMed] [Google Scholar]
- Berche P. A., North R. J. Non-H-2 restriction of expression of passively transferred delayed sensitivity. J Exp Med. 1982 May 1;155(5):1334–1343. doi: 10.1084/jem.155.5.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biozzi G., Asofsky R., Lieberman R., Stiffel C., Mouton D., Benacerraf B. Serum concentrations and allotypes of immunoglobulins in two lines of mice genetically selected for "high" or "low" antibody synthesis. J Exp Med. 1970 Oct 1;132(4):752–764. doi: 10.1084/jem.132.4.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biozzi G., Mouton D., Sant'Anna O. A., Passos H. C., Gennari M., Reis M. H., Ferreira V. C., Heumann A. M., Bouthillier Y., Ibanez O. M. Genetics of immunoresponsiveness to natural antigens in the mouse. Curr Top Microbiol Immunol. 1979;85:31–98. [PubMed] [Google Scholar]
- Biozzi G., Mouton D., Stiffel C., Bouthillier Y. A major role of the macrophage in quantitative genetic regulation of immunoresponsiveness and antiinfectious immunity. Adv Immunol. 1984;36:189–234. doi: 10.1016/s0065-2776(08)60902-5. [DOI] [PubMed] [Google Scholar]
- Byfield P. E., Howard J. G. Equivalent graft-versus-host reactivity of spleen cells from two lines of mice genetically selected for high and low humoral antibody formation. Transplantation. 1972 Jul;14(1):133–135. doi: 10.1097/00007890-197207000-00023. [DOI] [PubMed] [Google Scholar]
- Cannat A., Bousquet C., Serre A. Response of high and low antibody producer to Brucella. Ann Immunol (Paris) 1978 Jul-Sep;129 100(5):669–683. [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. doi: 10.1128/iai.19.3.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuensalida-Draper E. Chlamydia psittaci infection in lines of mice with high or low antibody responses. Ann Immunol (Paris) 1980 Sep-Oct;131D(2):217–222. [PubMed] [Google Scholar]
- Hale C., Howard J. G. Immunological regulation of experimental cutaneous leishmaniasis. 2. Studies with Biozzi high and low responder lines of mice. Parasite Immunol. 1981 Spring;3(1):45–55. doi: 10.1111/j.1365-3024.1981.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Lagrange P. H., Hurtrel B., Thickstun P. M. Immunological behavior after mycobacterial infection in selected lines of mice with high or low antibody responses. Infect Immun. 1979 Jul;25(1):39–47. doi: 10.1128/iai.25.1.39-47.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liacopoulos-Briot M., Bouthillier Y., Mouton D., Lambert F., Decreusefond C., Stiffel C., Biozzi G. Comparison of skin allograft rejection and cytotoxic antibody production in two lines of mice genetically selected for "high" and "low" antibody synthesis. Transplantation. 1972 Nov;14(5):590–596. doi: 10.1097/00007890-197211000-00010. [DOI] [PubMed] [Google Scholar]
- Liacopoulos-Briot M., Mouton D., Stiffel C., Lambert F., Decreusefond C., Bouthillier Y., Biozzi G. Réponse a la phytohémagglutinine des lymphocytes de deux lignées de souris génétiquement sélectionnées pour une forte et une faible synthèse d'anticorps. Ann Immunol (Paris) 1974 Mar-Apr;125(3):393–404. [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Berche P. A., Newborg M. F. Immunologic consequences of antibiotic-induced abridgement of bacterial infection: effect on generation and loss of protective T cells and level of immunologic memory. J Immunol. 1981 Jul;127(1):342–346. [PubMed] [Google Scholar]
- North R. J. Cellular kinetics associated with the development of acquired cellular resistance. J Exp Med. 1969 Aug 1;130(2):299–314. doi: 10.1084/jem.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Deissler J. F. Nature of "memory" in T-cell mediated antibacterial immunity: cellular parameters that distinguish between the active immune response and a state of "memory". Infect Immun. 1975 Oct;12(4):761–767. doi: 10.1128/iai.12.4.761-767.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Nature of "memory" in T-cell-mediated antibacterial immunity: anamnestic production of mediator T cells. Infect Immun. 1975 Oct;12(4):754–760. doi: 10.1128/iai.12.4.754-760.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman S. F. Antigen recognition: the specificity of T cells involved in the cellular immune response. Transplant Rev. 1972;10:97–111. doi: 10.1111/j.1600-065x.1972.tb01540.x. [DOI] [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A., Sachs D. H. Resistance to Listeria monocytogenes in mice: genetic control by genes that are not linked to the H-2 complex. J Infect Dis. 1979 Feb;139(2):228–231. doi: 10.1093/infdis/139.2.228. [DOI] [PubMed] [Google Scholar]
- Wiener E., Bandieri A. Differences in antigen handling by peritoneal macrophages from the Biozzi high and low responder lines of mice. Eur J Immunol. 1974 Jul;4(7):457–463. doi: 10.1002/eji.1830040703. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Blanden R. V., Langman R. E. Early appearance of sensitized lymphocytes in mice infected with Listeria monocytogenes. J Immunol. 1974 Feb;112(2):496–501. [PubMed] [Google Scholar]


