Abstract
Leishmania species engineered to express high levels of the surface metalloprotease gp63 have enhanced capacity of migration through extracellular matrix in vitro. This correlates with gp63 degradation of extracellular matrix components, such as collagen type IV and fibronectin, and suggests an important role for gp63 in the pathogenesis of leishmaniasis.
Leishmania species are digenetic protozoa that alternately parasitize their sand fly vectors and mammalian macrophages. Parasites are deposited in the mammalian skin by infected sand flies and thereafter must interact with and overcome a variety of obstacles, including extracellular matrix (ECM) and basement membrane (BM) proteins, to establish infection within macrophage phagolysosomes (6). The 63-kDa glycoprotein gp63 is a zinc-dependent metalloprotease found on the surface of the parasite that facilitates complement inactivation in serum (3), interaction with the host macrophage (2, 10, 11) and intraphagolysosomal survival (8, 11). Structural and biochemical similarities exist between gp63 and members of the matrix metalloproteases (4, 14). The latter are important for enhancing the migration of some tumor cells through the ECM and BM, aiding in their metastasis (15, 16).
To test the hypothesis that gp63 facilitates parasite migration through the ECM, we employed a commercial invasion system in which parasites are placed in a cell culture insert and assessed for their ability to pass through the insert's ECM (Matrigel)-impregnated 8-μm-diameter pores (Becton Dickinson, Franklin Lakes, N.J.) (16). An attenuated, gp63-deficient variant of Leishmania amazonensis (12) was transfected with the following plasmid constructs for use in this study: (i) pX, an episomal expression vector; (ii) pX-gp63, which expresses wild-type gp63; and (iii) pX-E265D, which expresses an equivalent level of a proteolytically inactive form of gp63 (12). Stationary-phase promastigotes of each transfectant line were washed and suspended at a concentration of 108 cells ml−1 in Hanks buffered saline solution (Invitrogen, Carlsbad, Calif.). One milliliter of each parasite solution was applied to two cell culture inserts, one embedded with Matrigel and the other without Matrigel (control insert). At increasing times postinoculation, the numbers of parasites present in the lower wells were determined and the percentage of migration was calculated by dividing the number of parasites migrating through the inserts containing Matrigel by the number migrating through the control inserts. No differences were seen in the migrations of different transfectants across control inserts, with complete equilibration of parasite density achieved within 20 min.
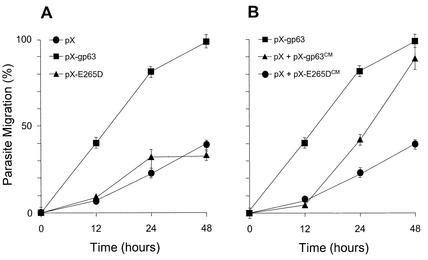
Migration was assessed over a 48-h period (Fig. 1A). Approximately 40% of the pX-gp63 promastigotes had migrated into the lower chamber at 12 h after inoculation, while only 7% of pX transfectants had migrated by this time. At 24 and 48 h after inoculation, the differences were even more pronounced, with 80 and nearly 100% of the pX-gp63 transfectants, respectively, having migrated across the Matrigel, compared with 22 and 40% of the pX transfectants, respectively.
FIG. 1.
Leishmania migration through the ECM is enhanced by expression of active gp63. (A) Gp63-deficient Leishmania transfected with the pX expression vector (pX) or constructs expressing active gp63 (pX-gp63) or a proteolytically inactive gp63 mutant (pX-E265D) were tested for their capacity to migrate across a Matrigel matrix as described in the text. (B) Migration of the pX transfectant (pX-gp63) across a Matrigel matrix was assessed after incubation, in conditioned medium, of lines expressing active gp63 (pX + pX-gp63CM) or the proteolytically inactive gp63 mutant (pX + pX-E265DCM). Migration of the pX-gp63 transfectant was monitored as a control. No significant differences in the migrations of the different cell types through control inserts lacking Matrigel were noted. Values (± standard errors of the means) from three separate experiments were compiled for this figure.
Since zinc is an essential cofactor for gp63 activity (8), we performed the assay under zinc-abundant and zinc-depleted conditions (data not shown). Zinc chelation was performed by preincubation of parasites, and conduction of the assay, in a 25 mM concentration of the extracellular zinc chelator bathophenanthroline. This had no deleterious effect on parasite viability during the time course of the experiment. Upon zinc depletion, the capacity of the pX-gp63 transfectants to migrate across the matrix was diminished by 60%, implicating gp63 in the enhancement of ECM migration in vitro.
To further test the specificity of the involvement of gp63 in ECM migration, we assessed the migration capacity of the pX-E265D line, which produces the inactive form of gp63, and found that these parasites migrated through the ECM at approximately the same rate as did the pX transfectants. These results strongly suggest that gp63 is involved in the migration process. The fact that the pX and pX-E265D lines migrated across the Matrigel matrix could be explained to some extent by the fact that the attenuated, gp63-deficient variant of L. amazonensis used in the study possesses a very small amount of active gp63 that could contribute to ECM degradation (12). In addition, other proteases or surface ligands present in all parasite stocks used could contribute to a basal capacity of parasites to migrate through ECM that may not directly involve gp63.
Our group recently reported that active gp63 is released from a wide variety of Leishmania species (13). To test whether released gp63 could potentiate parasite migration through the ECM, we suspended pX promastigotes in the conditioned medium of the pX-gp63 line (pX-gp63CM) or that of the pX-E265D line (pX-E265DCM) and then tested these parasites in the ECM migration assay (Fig. 1B) (13). Addition of pX-gp63CM enhanced the migration of pX parasites across the Matrigel matrix, with the migration becoming significantly greater than that afforded by pX-E265DCM by 24 h after inoculation. By 48 h, nearly as many pX and pX-gp63CM cells had migrated across the matrix as had pX-gp63 cells alone. pX parasites either in Hanks buffered saline solution or resuspended in their own conditioned medium showed the same migration (data not shown). These results clearly demonstrate that active gp63 released from Leishmania species has the capacity to promote the migration of parasites through the ECM. They also suggest that the effects of surface gp63 and secreted gp63 are additive in this regard.
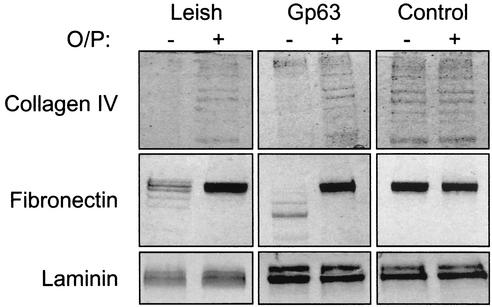
To correlate the ability of leishmanial gp63 to mediate ECM migration and its capacity to digest components of the ECM, we incubated approximately 10 μg each of purified collagen type IV, fibronectin, and laminin with approximately 1 μg of purified gp63 or 107 stationary-phase promastigotes that had been lightly fixed with glutaraldehyde (7, 12) (Fig. 2). Controls consisted of reaction mixtures containing protein substrate in buffer alone or those in which gp63 was inactivated by preincubation in a 25 mM concentration of the zinc chelator orthophenanthroline. Substrate degradation was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by staining with Coomassie blue. The gp63 from either source was effective in digesting collagen type IV. The preparations of intact collagen contained subunits of various lengths that resembled a ladder of protein bands as seen by SDS-PAGE. After incubation with gp63, the ladder effect was lost, reflecting digestion of the proteins into smaller units that became a smear of smaller proteins of less than 15 kDa (not shown). Control incubations of collagen either in buffer alone or in zinc-depleted conditions showed the preservation of the intact ladder of collagen subunits. Fibronectin appeared as an intact molecule by SDS-PAGE but was similarly digested into smaller protein units when incubated with gp63 from either source. Under zinc-depleted conditions, gp63 did not degrade fibronectin. Interestingly, the patterns of digested fibronectin seen by SDS-PAGE were somewhat different depending on the source of gp63 used. Cell-associated gp63 appeared to digest fibronectin into larger subunits than did purified gp63. This is not unexpected when membrane-bound and soluble proteases are compared under different conditions. Laminin appeared to be resistant to digestion by gp63 since it remained intact as protein subunits, regardless of the conditions used for incubation.
FIG. 2.
Surface-bound and purified gp63 can proteolyze ECM components. Purified gp63 and Leishmania promastigotes lightly fixed with glutaraldehyde were incubated with the ECM components fibronectin, collagen type IV, and laminin, and mixtures were analyzed by SDS-PAGE for evidence of degradation. Reactions were performed in the presence (+) or absence (−) of a 25 mM concentration of the zinc chelator orthophenanthroline (O/P).
We report here that Leishmania expressing high levels of active gp63 have enhanced capacity for migration through the ECM in vitro. This is further supported by the demonstration that gp63 can degrade ECM components. Since the ultimate goal of invading Leishmania is to become intracellular, we envision that enhanced migration at the site of inoculation may promote parasite binding to and phagocytosis by macrophages. In addition, migration through the ECM and BM may facilitate the access of parasites to the blood or lymph circulation for dissemination to distant sites (1, 9) where they may parasitize tissue macrophages. Additionally, attachment of Leishmania to native or degradation products of ECM proteins may also help facilitate parasite migration or host macrophage activation or migration (16). Further study is necessary to determine the relevance of ECM degradation to the facilitation of parasite dermal migration in vivo.
Acknowledgments
This work was supported an American Heart Association Physician-Scientist Postdoctoral Fellowship to B.S.M. and by NIH grants to D.M.E. and K.-P.C.
Editor: J. M. Mansfield
REFERENCES
- 1.Bandyopadhyay, K., S. Karmakar, A. Ghosh, and P. K. Das. 2002. High affinity binding between laminin and laminin binding protein of Leishmania is stimulated by zinc and may involve laminin zinc-finger like sequences. Eur. J. Biochem. 269:1622-1629. [DOI] [PubMed] [Google Scholar]
- 2.Brittingham, A., G. Chen, B. S. McGwire, K. P. Chang, and D. M. Mosser. 1999. Interaction of Leishmania gp63 with cellular receptors for fibronectin. Infect. Immun. 67:4477-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brittingham, A., C. J. Morrison, W. R. McMaster, B. S. McGwire, K. P. Chang, and D. M. Mosser. 1995. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J. Immunol. 155:3102-3111. [PubMed] [Google Scholar]
- 4.Button, L. L., G. Wilson, C. R. Astell, and W. R. McMaster. 1993. Recombinant Leishmania surface glycoprotein GP63 is secreted in the baculovirus expression system as a latent metalloproteinase. Gene 134:75-81. [DOI] [PubMed] [Google Scholar]
- 5.Chang, C. S., and K. P. Chang. 1986. Monoclonal antibody affinity purification of a Leishmania membrane glycoprotein and its inhibition of leishmania-macrophage binding. Proc. Natl. Acad. Sci. USA 83:100-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, K. P., G. Chaudhuri, and D. Fong. 1990. Molecular determinants of Leishmania virulence. Annu. Rev. Microbiol. 44:499-529. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhuri, G., and K. P. Chang. 1988. Acid protease activity of a major surface membrane glycoprotein (gp63) from Leishmania mexicana promastigotes. Mol. Biochem. Parasitol. 27:43-52. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhuri, G., M. Chaudhuri, A. Pan, and K. P. Chang. 1989. Surface acid proteinase (gp63) of Leishmania mexicana. A metalloenzyme capable of protecting liposome-encapsulated proteins from phagolysosomal degradation by macrophages. J. Biol. Chem. 264:7483-7489. [PubMed] [Google Scholar]
- 9.Ghosh, A., K. Bandyopadhyay, L. Kole, and P. K. Das. 1999. Isolation of a laminin-binding protein from the protozoan parasite Leishmania donovani that may mediate cell adhesion. Biochem. J. 337:551-558. [PMC free article] [PubMed] [Google Scholar]
- 10.Liu, X., and K. P. Chang. 1992. Extrachromosomal genetic complementation of surface metalloproteinase (gp63)-deficient Leishmania increases their binding to macrophages. Proc. Natl. Acad. Sci. USA 89:4991-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGwire, B., and K. P. Chang. 1994. Genetic rescue of surface metalloproteinase (gp63)-deficiency in Leishmania amazonensis variants increases their infection of macrophages at the early phase. Mol. Biochem. Parasitol. 66:345-347. [DOI] [PubMed] [Google Scholar]
- 12.McGwire, B. S., and K. P. Chang. 1996. Posttranslational regulation of a Leishmania HEXXH metalloprotease (gp63). The effects of site-specific mutagenesis of catalytic, zinc binding, N-glycosylation, and glycosyl phosphatidylinositol addition sites on N-terminal end cleavage, intracellular stability, and extracellular exit. J. Biol. Chem. 271:7903-7909. [DOI] [PubMed] [Google Scholar]
- 13.McGwire, B. S., W. A. O'Connell, K. P. Chang, and D. M. Engman. 2002. Extracellular release of the glycosylphosphatidylinositol (GPI)-linked Leishmania surface metalloprotease, gp63, is independent of GPI phospholipolysis: implications for parasite virulence. J. Biol. Chem. 277:8802-8809. [DOI] [PubMed] [Google Scholar]
- 14.Stocker, W., F. Grams, U. Baumann, P. Reinemer, F. X. Gomis-Ruth, D. B. McKay, and W. Bode. 1995. The metzincins—topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci. 4:823-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, M., and M. E. Stearns. 1988. Blocking of collagenase secretion by estramustine during in vitro tumor cell migration. Cancer Res. 48:6262-6271. [PubMed] [Google Scholar]
- 16.Yu, Q., and I. Stamenkovic. 2000. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor migration and angiogenesis. Genes Dev. 14:163-176. [PMC free article] [PubMed] [Google Scholar]