Abstract
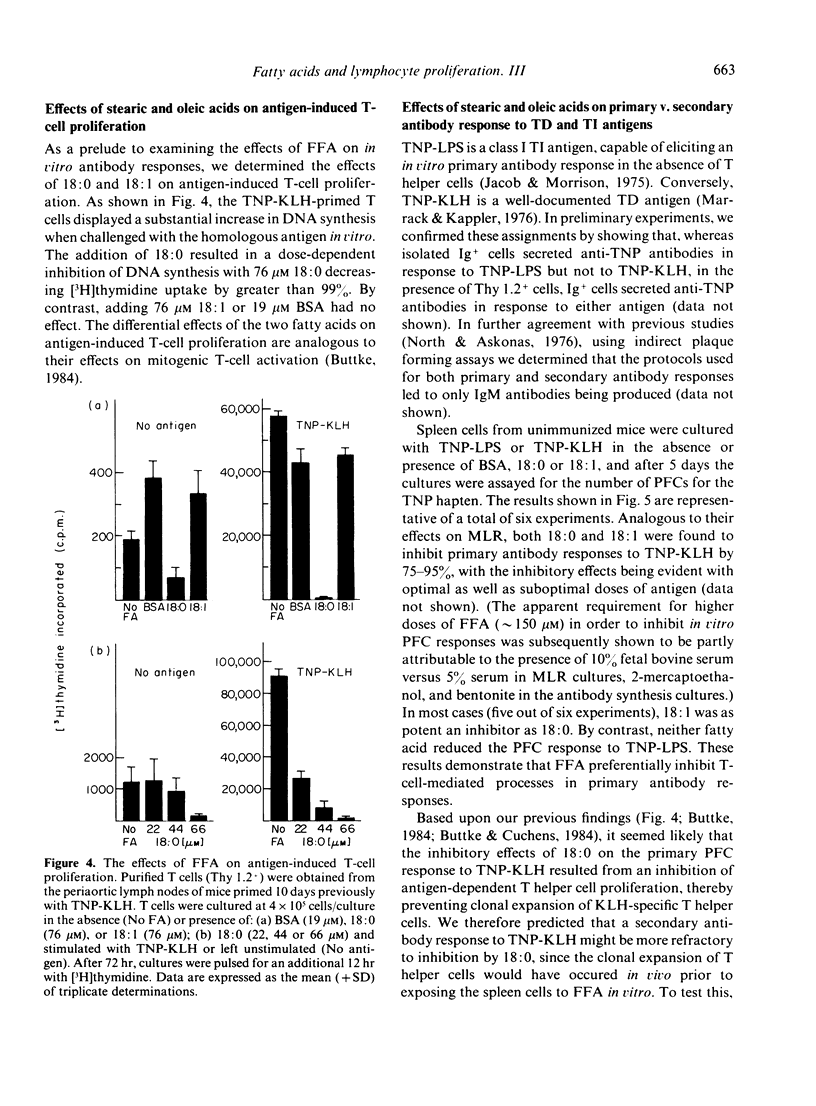
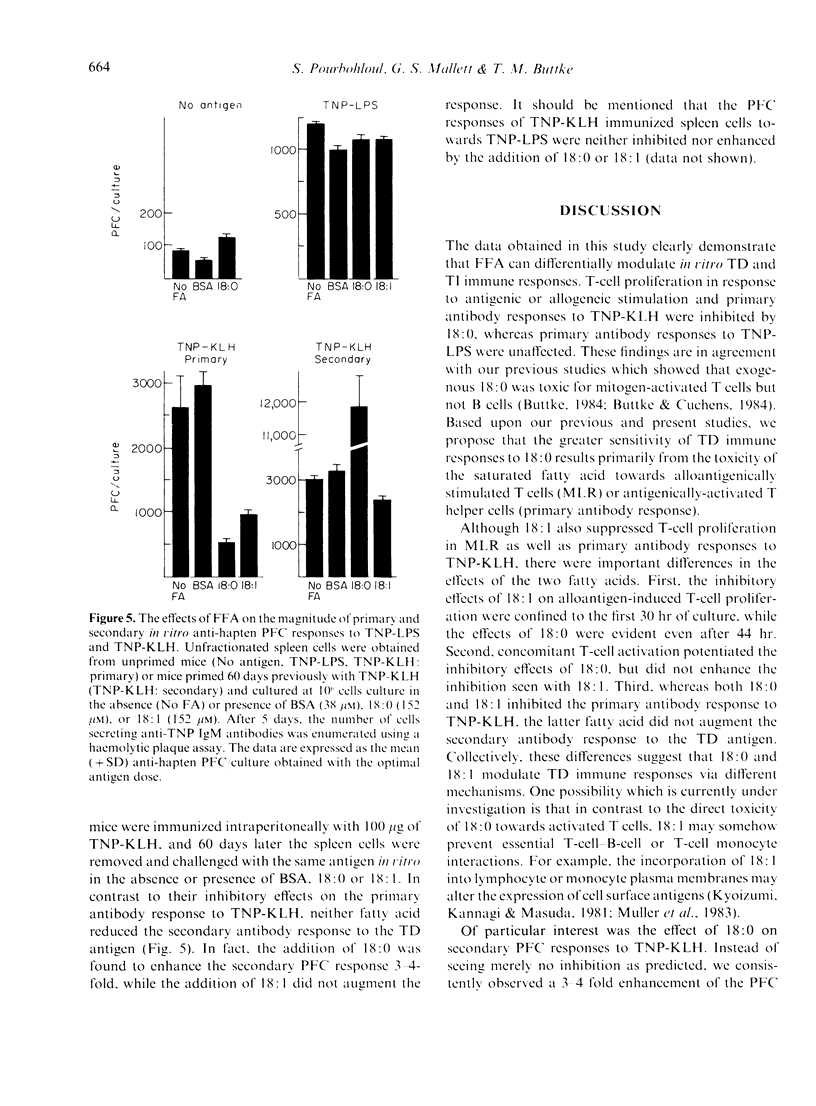
Free fatty acids (FFA) were tested for their effects on in vitro thymus-dependent (TD) and thymus-independent (TI) immune responses. Murine T cells proliferating in one-way mixed leucocyte reactions (MLR) were extremely sensitive to inhibition by exogenous stearic acid (18:0) but were only moderately affected by oleic acid (18:1). T-cell proliferation was suppressed when 18:0 was added as late as 44 hr after allogeneic stimulation, but sensitivity to 18:1 was limited to the first 30 hr of culture. The inhibitory effects of 18:0, but not 18:1 were potentiated by concomitant T-cell activation and under such conditions the effects of 18:0 were irreversible within 5 hr. The two fatty acids were additionally tested for their effects on the anti-hapten antibody-secreting cell responses to TD and TI antigens. Both 18:0 and 18:1 inhibited the primary antibody response elicited by a TD antigen (TNP-KLH) but neither fatty acid significantly affected the primary antibody response to a TI antigen (TNP-LPS). Following in vivo immunization with TNP-KLH, isolated spleen cells were challenged with the same antigen in vitro in the presence of FFA. Whereas 18:1 had little effect on the secondary immune response, the addition of 18:0 led to a 3-4-fold increase in the number of anti-TNP plaque forming cells. Further studies showed that TNP-KLH-induced T cell proliferation was potently inhibited by 18:0 but 18:1 had no effect. These results suggest that an inhibition of T-cell proliferation is the primary way in which 18:0 modulates TD immune responses in vitro. By contrast, 18:1 appears to inhibit primary antibody responses and MLR via alternative mechanisms.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Miller A., Sercarz E. E. The fine specificity of regulatory T cells. I. Hen egg-white lysozyme-induced suppressor T cells in a genetically nonresponder mouse strain do not recognize a closely related immunogenic lysozyme. J Immunol. 1979 Mar;122(3):871–877. [PubMed] [Google Scholar]
- Buttke T. M., Cuchens M. A. Inhibition of lymphocyte proliferation by free fatty acids. II. Toxicity of stearic acid towards phytohaemagglutinin-activated T cells. Immunology. 1984 Nov;53(3):507–514. [PMC free article] [PubMed] [Google Scholar]
- Buttke T. M., Mallett G. S., Cuchens M. A. Positive selection of mouse B and T lymphocytes and analysis of isolated populations by flow cytometry. J Immunol Methods. 1983 Mar 11;58(1-2):193–207. doi: 10.1016/0022-1759(83)90275-2. [DOI] [PubMed] [Google Scholar]
- Chan E. L., Henry C. Coexistence of helper and suppressor activities in carrier-primed spleen cells. J Immunol. 1976 Oct;117(4):1132–1138. [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Cowing C., Chapdelaine J. M. T cells discriminate between Ia antigens expressed on allogeneic accessory cells and B cells: a potential function for carbohydrate side chains on Ia molecules. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6000–6004. doi: 10.1073/pnas.80.19.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M. T cell suppression in vitro. I. Role in regulation of antibody responses. Eur J Immunol. 1974 Oct;4(10):660–666. doi: 10.1002/eji.1830041005. [DOI] [PubMed] [Google Scholar]
- Gallily R., Garvey J. S. Primary stimulation of rats and mice with hemocyanin in soluton and adsorbed on bentonite. J Immunol. 1968 Nov;101(5):924–929. [PubMed] [Google Scholar]
- Gill R., Clark W. Membrane structure-function relationships in cell-mediated cytolysis. I. Effect of exogenously incorporated fatty acids on effector cell function in cell-mediated cytolysis. J Immunol. 1980 Aug;125(2):689–695. [PubMed] [Google Scholar]
- Hawley H. P., Gordon G. B. The effects of long chain free fatty acids on human neutrophil function and structure. Lab Invest. 1976 Feb;34(2):216–222. [PubMed] [Google Scholar]
- Jacobs D. M., Morrison D. C. Stimulation of a T-independent primary anti-hapten response in vitro by TNP-lipopolysaccharide (TNP-LPS). J Immunol. 1975 Jan;114(1 Pt 2):360–364. [PubMed] [Google Scholar]
- Klausner R. D., Bhalla D. K., Dragsten P., Hoover R. L., Karnovsky M. J. Model for capping derived from inhibition of surface receptor capping by free fatty acids. Proc Natl Acad Sci U S A. 1980 Jan;77(1):437–441. doi: 10.1073/pnas.77.1.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyoizumi S., Kannagi R., Masuda T. Membrane expression of Fc-receptors in cultured leukemic cell lines. I. Induction of Fc-receptor in undifferentiated types of cells after passive modulation of lipid viscosity. J Immunol. 1981 Dec;127(6):2252–2256. [PubMed] [Google Scholar]
- Mahoney E. M., Hamill A. L., Scott W. A., Cohn Z. A. Response of endocytosis to altered fatty acyl composition of macrophage phospholipids. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4895–4899. doi: 10.1073/pnas.74.11.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. W. Antigen-specific and nonspecific mediatiors of T cell/B cell cooperation. II. Two helper T cells distinguished by their antigen sensitivities. J Immunol. 1976 May;116(5):1373–1378. [PubMed] [Google Scholar]
- Miller N. W., Clem L. W. Microsystem for in vitro primary and secondary immunization of channel catfish (Ictalurus punctatus) leukocytes with hapten-carrier conjugates. J Immunol Methods. 1984 Sep 4;72(2):367–379. doi: 10.1016/0022-1759(84)90006-1. [DOI] [PubMed] [Google Scholar]
- Muller C. P., Stephany D. A., Shinitzky M., Wunderlich J. R. Changes in cell-surface expression of MHC and Thy-1.2 determinants following treatment with lipid modulating agents. J Immunol. 1983 Sep;131(3):1356–1362. [PubMed] [Google Scholar]
- North J. R., Askonas B. A. IgG response in vitro. I. The requirement for an intermediate responsive cell type. Eur J Immunol. 1976 Jan;6(1):8–15. doi: 10.1002/eji.1830060104. [DOI] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Sercarz E. E., Yowell R. L., Turkin D., Miller A., Araneo B. A., Adorini L. Different functional specificity repertoires for suppressor and helper T cells. Immunol Rev. 1978;39:108–136. doi: 10.1111/j.1600-065x.1978.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Singer A., Cowing C., Hathcock K. S., Dickler H. B., Hodes R. J. Cellular and genetic control of antibody responses in vitro. III. Immune response gene regulation of accessory cell function. J Exp Med. 1978 Jun 1;147(6):1611–1620. doi: 10.1084/jem.147.6.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Takemori T. Selective roles of thymus-derived lymphocytes in the antibody response. I. Differential suppressive effect of carrier-primed T cells on hapten-specific IgM and IgG antibody responses. J Exp Med. 1974 Jul 1;140(1):239–252. doi: 10.1084/jem.140.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyman C., Morgan S. J., Belin J., Smith A. D. Phytohaemagglutinin stimulation of human lymphocytes: effect of fatty acids on uridine uptake and phosphoglyceride fatty acid profile. Biochim Biophys Acta. 1977 Jan 24;496(1):155–166. doi: 10.1016/0304-4165(77)90123-4. [DOI] [PubMed] [Google Scholar]


