Abstract
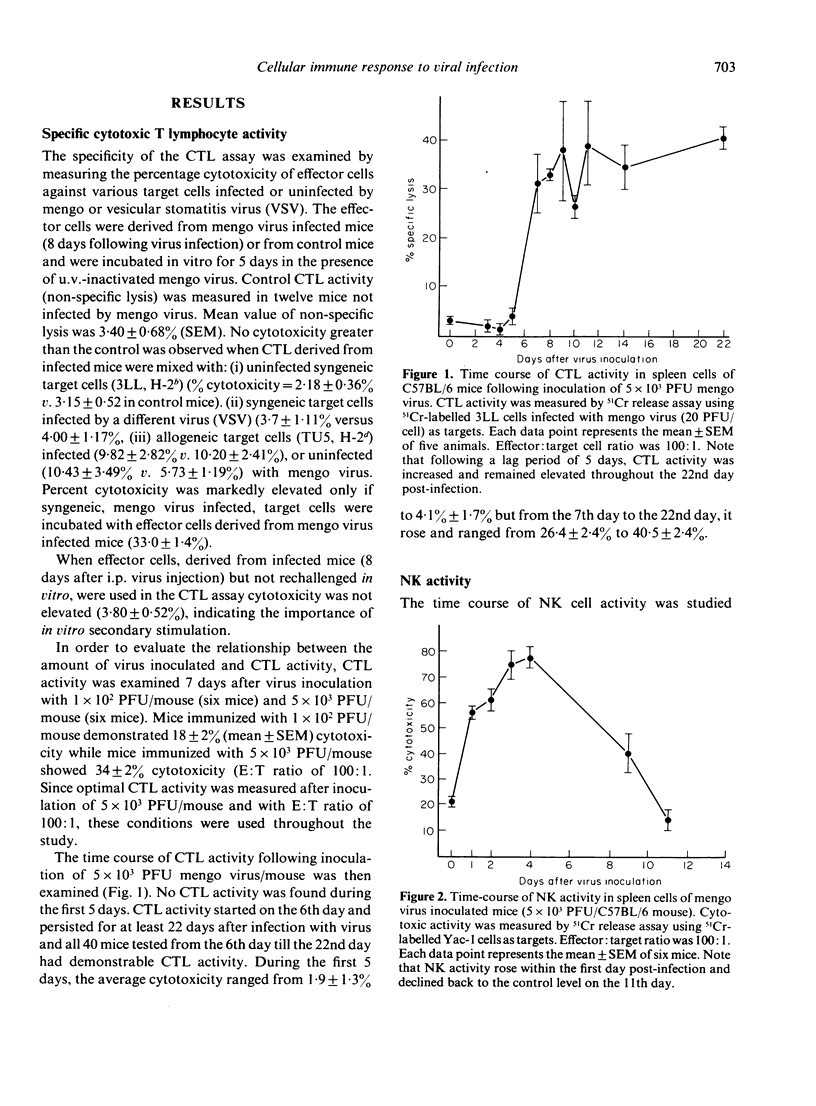
Inbred C57BL/6 mice were inoculated intraperitoneally (i.p.) with mengo virus. The activity of cytotoxic T lymphocytes (CTL) and natural killer (NK) cells were measured during the first 22 days following infection. The CTL response began 7 days after virus inoculation, persisted for at least 22 days and was related to the dose of the virus inoculated. NK cell activity was elevated within 24 hr, reached its peak level on the fourth day and declined to normal levels on the eleventh day after exposure to the virus. These results suggest that NK cells represent the first cellular immune response to restrict mengo virus spread while specific CTL appear later and are probably responsible for further restriction, elimination and prevention of the viral disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel M. J., Shek W. R., Summers B. A. Lymphocyte-mediated immune cytotoxicity in dogs infected with virulent canine distemper virus. Infect Immun. 1982 Aug;37(2):592–600. doi: 10.1128/iai.37.2.592-600.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft G. J., Shellam G. R., Chalmer J. E. Genetic influences on the augmentation of natural killer (NK) cells during murine cytomegalovirus infection: correlation with patterns of resistance. J Immunol. 1981 Mar;126(3):988–994. [PubMed] [Google Scholar]
- Biron C. A., Welsh R. M. Blastogenesis of natural killer cells during viral infection in vivo. J Immunol. 1982 Dec;129(6):2788–2795. [PubMed] [Google Scholar]
- Bukowski J. F., Woda B. A., Habu S., Okumura K., Welsh R. M. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983 Sep;131(3):1531–1538. [PubMed] [Google Scholar]
- Ciavarra R., Kang C. Y., Forman J. Mechanisms for generating cell membrane antigens that are recognized by cytotoxic T lymphocytes. Fed Proc. 1981 Feb;40(2):222–227. [PubMed] [Google Scholar]
- Clark E. A., Harmon R. C. Genetic control of natural cytotoxicity and hybrid resistance. Adv Cancer Res. 1980;31:227–285. doi: 10.1016/s0065-230x(08)60659-4. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Bennink J. R. Monitoring the integrity of self: biology of MHC-restriction of virus-immune T cells. Fed Proc. 1981 Feb;40(2):218–221. [PubMed] [Google Scholar]
- Finberg R., Benacerraf B. Induction, control and consequences of virus specific cytotoxic T cells. Immunol Rev. 1981;58:157–180. doi: 10.1111/j.1600-065x.1981.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Heremans H., Billiau A., De Somer P. Interferon in experimental viral infections in mice: tissue interferon levels resulting from the virus infection and from exogenous interferon therapy. Infect Immun. 1980 Nov;30(2):513–522. doi: 10.1128/iai.30.2.513-522.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Job L. P., Woodruff J. F. Lysis of infected myofibers by coxsackievirus B-3-immune T lymphocytes. Am J Pathol. 1980 Mar;98(3):681–694. [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Engler H., Zawatzky R., Schindler L. Role of natural killer cells and of interferon in natural resistance against virus infections. Adv Exp Med Biol. 1982;155:785–797. doi: 10.1007/978-1-4684-4394-3_86. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- Pfau C. J., Valenti J. K., Jacobson S., Pevear D. C. Cytotoxic T cells are induced in mice infected with lymphocytic choriomeningitis virus strains of markedly different pathogenicities. Infect Immun. 1982 May;36(2):598–602. doi: 10.1128/iai.36.2.598-602.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnan G. V., Manischewitz J. E. The role of natural killer cells and antibody-dependent cell-mediated cytotoxicity during murine cytomegalovirus infection. J Exp Med. 1979 Dec 1;150(6):1549–1554. doi: 10.1084/jem.150.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler L., Engler H., Kirchner H. Activation of natural killer cells and induction of interferon after injection of mouse hepatitis virus type 3 in mice. Infect Immun. 1982 Mar;35(3):869–873. doi: 10.1128/iai.35.3.869-873.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med. 1978 Jul 1;148(1):163–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Zinkernagel R. M. Heterospecific cytotoxic cell activity induced during the first three days of acute lymphocytic choriomeningitis virus infection in mice. Nature. 1977 Aug 18;268(5621):646–648. doi: 10.1038/268646a0. [DOI] [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus B-3 infection. I. Model and viral specificity1. J Immunol. 1977 Apr;118(4):1159–1164. [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974 Dec;113(6):1726–1734. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Major transplantation antigens, viruses, and specificity of surveillance T cells. Contemp Top Immunobiol. 1977;7:179–220. doi: 10.1007/978-1-4684-3054-7_5. [DOI] [PubMed] [Google Scholar]


