Abstract
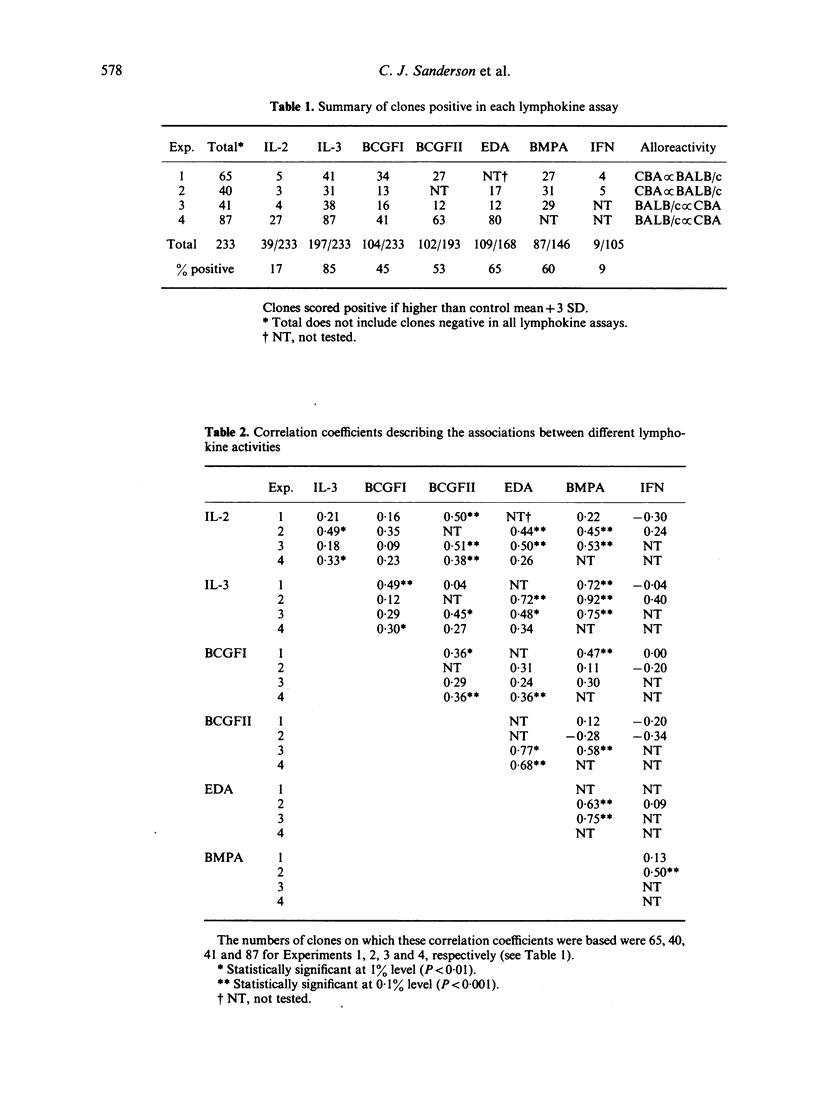
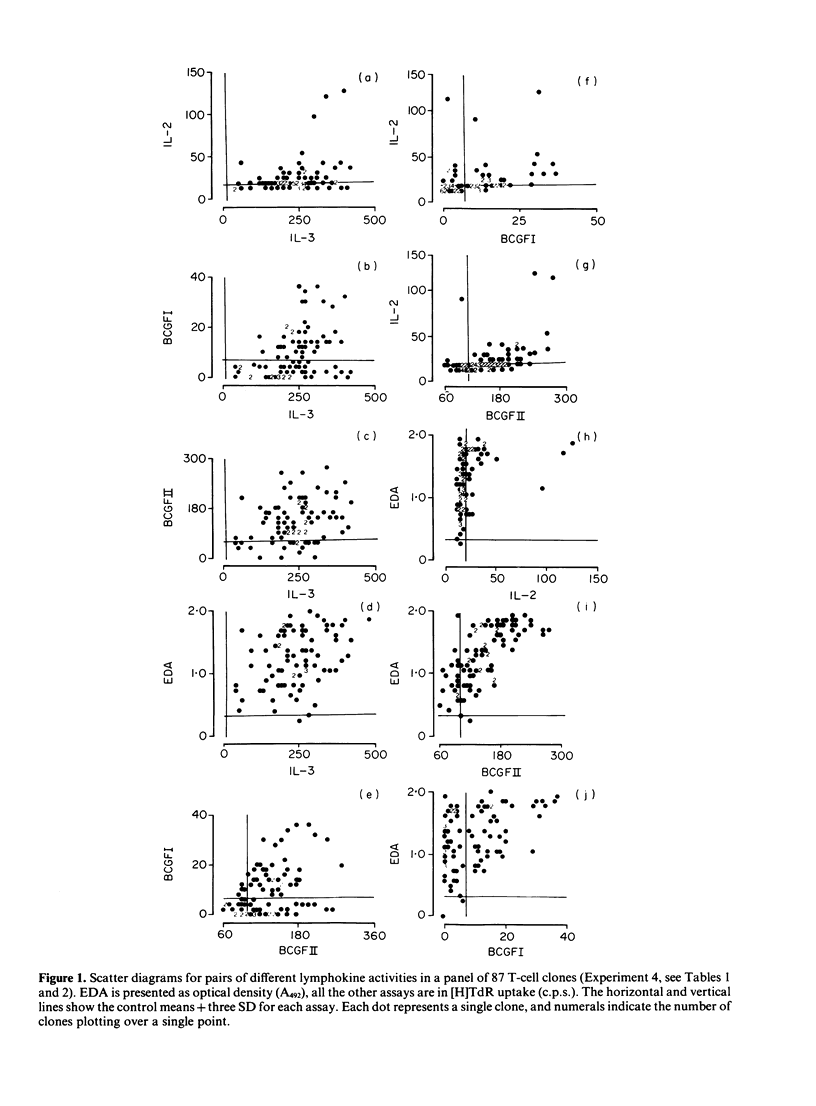
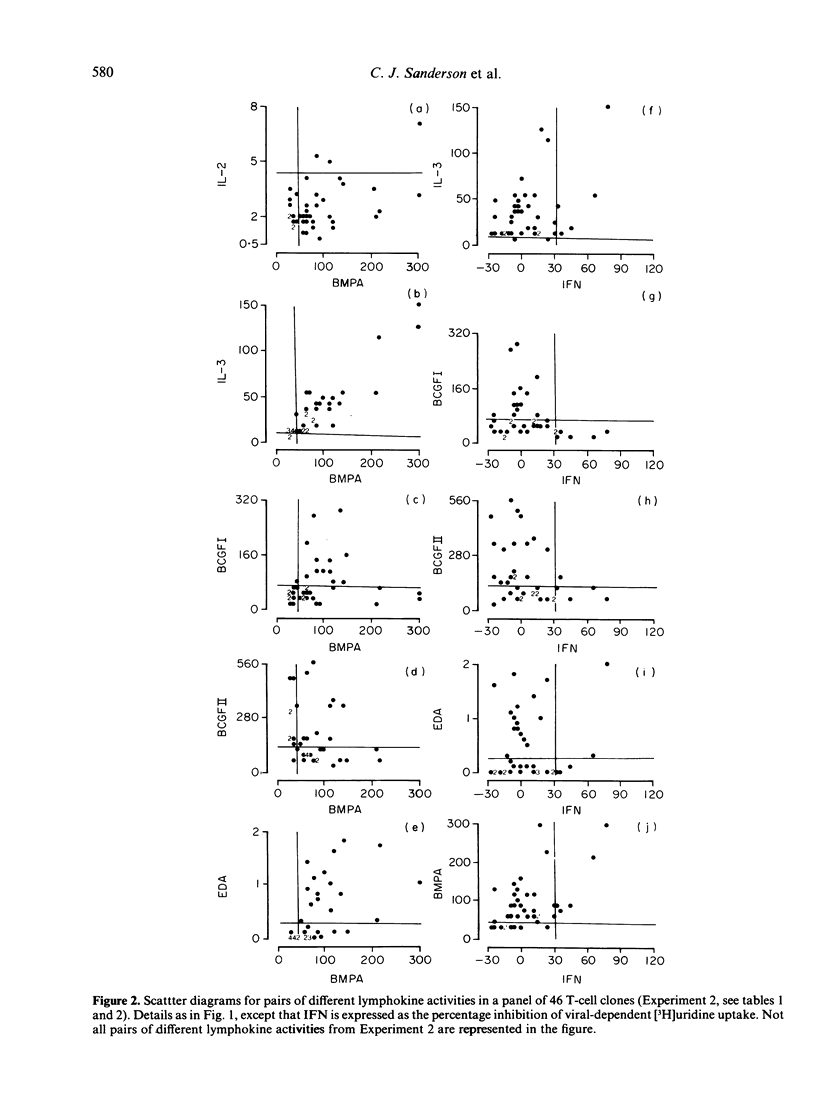
A total of 233 primary alloreactive T-cell clones have been tested for the production of interleukin-2 (IL-2), interleukin-3 (IL-3), immune(gamma) interferon (IFN) and granulocyte-macrophage colony-stimulating factor (CSF-2), B-cell growth factor I and II (BCGFI, BCGFII), and eosinophil differentiation factor (EDF). EDF was assayed by means of the eosinophil differentiation assay (EDA). Two principal correlations were observed: IL-3 was shown to be the major lymphokine detected in the bone marrow proliferation assay (BMPA) used to detect CSF-2, and there was a high correlation between the EDA and BCGFII. Subsequent work has suggested that this latter correlation is because a single factor is responsible for both activities. Apart from these two exceptions, and low level correlations probably due to the fact that different assays detect more than one lymphokine, there was no evidence for co-ordinate expression of lymphokines. There was a large variation in amounts of individual lymphokines produced. More clones produced multiple lymphokines than would be expected from independent control. Taken together, this pattern of regulation is consistent with the hypothesis that antigen stimulation of T cells results in the activation of all the lymphokine genes, but the amount of each produced is determined by secondary controlling mechanisms.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. T., Giron D. J. Rapid sensitive assay for interferons based on the inhibition of MM virus nucleic acid synthesis. Appl Microbiol. 1970 Sep;20(3):317–322. doi: 10.1128/am.20.3.317-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayen A., Parkhouse R. M. Preparation and properties of a cytotoxic monoclonal rat anti-mouse Thy-1 antibody. J Immunol Methods. 1982;49(1):17–23. doi: 10.1016/0022-1759(82)90362-3. [DOI] [PubMed] [Google Scholar]
- Dutton R. W., Wetzel G. D., Swain S. L. Partial purification and characterization of a BCGFII from EL4 culture supernatants. J Immunol. 1984 May;132(5):2451–2456. [PubMed] [Google Scholar]
- Ely J. M., Prystowsky M. B., Eisenberg L., Quintans J., Goldwasser E., Glasebrook A. L., Fitch F. W. Alloreactive cloned T cell lines. V. Differential kinetics of IL 2, CSF, and BCSF release by a cloned T amplifier cell and its variant. J Immunol. 1981 Dec;127(6):2345–2349. [PubMed] [Google Scholar]
- Farrar J. J., Fuller-Farrar J., Simon P. L., Hilfiker M. L., Stadler B. M., Farrar W. L. Thymoma production of T cell growth factor (Interleukin 2). J Immunol. 1980 Dec;125(6):2555–2558. [PubMed] [Google Scholar]
- Fung M. C., Hapel A. J., Ymer S., Cohen D. R., Johnson R. M., Campbell H. D., Young I. G. Molecular cloning of cDNA for murine interleukin-3. Nature. 1984 Jan 19;307(5948):233–237. doi: 10.1038/307233a0. [DOI] [PubMed] [Google Scholar]
- Fuse A., Fujita T., Yasumitsu H., Kashima N., Hasegawa K., Taniguchi T. Organization and structure of the mouse interleukin-2 gene. Nucleic Acids Res. 1984 Dec 21;12(24):9323–9331. doi: 10.1093/nar/12.24.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Glasebrook A. L., Fitch F. W. Alloreactive cloned T cell lines. I. Interactions between cloned amplifier and cytolytic T cell lines. J Exp Med. 1980 Apr 1;151(4):876–895. doi: 10.1084/jem.151.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak H., Turner A. R., Shaw A. R., Yau O. W. Stimulation of [3H]thymidine uptake in mouse marrow by granulocyte-macrophage colony stimulating factor from mouse lung conditioned medium. J Immunol Methods. 1983 Jan 28;56(2):253–260. doi: 10.1016/0022-1759(83)90417-9. [DOI] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Nakanishi K., Paul W. E. B cell growth and differentiation factors. Immunol Rev. 1984 Apr;78:185–210. doi: 10.1111/j.1600-065x.1984.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Kelso A., Glasebrook A. L. Secretion of interleukin 2, macrophage-activating factor, interferon, and colony-stimulating factor by alloreactive T lymphocyte clones. J Immunol. 1984 Jun;132(6):2924–2931. [PubMed] [Google Scholar]
- Kelso A., MacDonald H. R., Smith K. A., Cerottini J. C., Brunner K. T. Interleukin 2 enhancement of lymphokine secretion by T lymphocytes: analysis of established clones and primary limiting dilution microcultures. J Immunol. 1984 Jun;132(6):2932–2938. [PubMed] [Google Scholar]
- Kelso A., Macdonald H. R. Precursor frequency analysis of lymphokine-secreting alloreactive T lymphocytes. Dissociation of subsets producing interleukin 2, macrophage-activating factor, and granulocyte-macrophage colony-stimulating factor on the basis of Lyt-2 phenotype. J Exp Med. 1982 Nov 1;156(5):1366–1379. doi: 10.1084/jem.156.5.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp M. R., Severinson-Gronowicz E., Schröder J., Strober S. Characterization of a spontaneous murine B cell leukemia (BCL1). II. Tumor cell proliferation and IgM secretion after stimulation by LPS. J Immunol. 1979 Sep;123(3):1000–1006. [PubMed] [Google Scholar]
- Miller R. A., Stutman O. Limiting dilution analysis of T helper cell heterogeneity: a single class of T cell makes both IL 2 and IL 3. J Immunol. 1983 Apr;130(4):1749–1753. [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Prystowsky M. B., Ely J. M., Beller D. I., Eisenberg L., Goldman J., Goldman M., Goldwasser E., Ihle J., Quintans J., Remold H. Alloreactive cloned T cell lines. VI. Multiple lymphokine activities secreted by helper and cytolytic cloned T lymphocytes. J Immunol. 1982 Dec;129(6):2337–2344. [PubMed] [Google Scholar]
- Sanderson C. J., Strath M. Isolation of specific antigen-reactive T-cell clones from nude (nu/nu) mice infected with Mesocestoides corti. Immunology. 1985 Feb;54(2):275–279. [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., Warren D. J., Strath M. Identification of a lymphokine that stimulates eosinophil differentiation in vitro. Its relationship to interleukin 3, and functional properties of eosinophils produced in cultures. J Exp Med. 1985 Jul 1;162(1):60–74. doi: 10.1084/jem.162.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. D. Biology and biochemistry of T cell-derived lymphokines. I. The coordinate synthesis of interleukin 2 and colony-stimulating factors in a murine T cell lymphoma. J Immunol. 1983 Jul;131(1):293–297. [PubMed] [Google Scholar]
- Zlotnik A., Roberts W. K., Vasil A., Blumenthal E., Larosa F., Leibson H. J., Endres R. O., Graham S. D., Jr, White J., Hill J. Coordinate production by a T cell hybridoma of gamma interferon and three other lymphokine activities: multiple activities of a single lymphokine? J Immunol. 1983 Aug;131(2):794–800. [PubMed] [Google Scholar]



