Abstract
The mechanisms by which Cryptococcus neoformans persists in an immunocompetent host are not well understood. Using a rat model of persistent infection, we investigated the ability of pulmonary macrophages (PuM) to phagocytize C. neoformans and produce monocyte chemotactic protein 1 (MCP-1) as a function of the length of time of infection and opsonin. The ability of macrophages to affect serum-mediated phagocytosis varied over the course of infection and was dependent on CD11b/c and CD18 expression. Infection resulted in increased MCP-1 levels within the lung, though the actual amounts varied over the course of infection. Immunohistochemical studies localized MCP-1 expression to macrophages and epithelioid cells. Enhanced production of MCP-1 by PuM from infected rats was confirmed by ex vivo studies. Induction of MCP-1 following serum-mediated phagocytosis was observed for PuM from both infected and noninfected rats and depended on the interaction of C. neoformans with CD11b/c and CD18. Specific antibody was more efficient than serum in promoting phagocytosis and consistently elicited more MCP-1. The relative amount of MCP-1 produced in association with phagocytosis was similar for PuM at all lengths of time of infection. Decreased MCP-1 production was observed for PuM obtained from older rats, including long-term (8 to 10 months)-infected and age-matched controls, suggesting that aging may affect the production of MCP-1 by PuM in response to cryptococcal infection. In summary, our results show that macrophages are an important source of MCP-1 during pulmonary cryptococcosis and that MCP-1 production is actively regulated during infection. Furthermore, we find that phagocytosis of C. neoformans can serve as an important stimulus for MCP-1 production by PuM, though the efficiency of this process is dependent on the opsonin type and may be affected by aging.
An increasing body of evidence suggests that exposure to Cryptococcus neoformans is common among immunocompetent individuals and that symptomatic cryptococcosis is often the result of reactivation of a latent focus of infection within the lung (8, 12, 15). To gain further insight into the states of acute infection, resolution, and latency, it is essential to have animal models of cryptococcosis that approximate the course of human infection. We have developed a rat model of persistent pulmonary cryptococcal infection that shares many features of pulmonary cryptococcosis in immunocompetent humans. In this model, fungal burden decreases within 1 month of infection in association with extensive granuloma formation and enhanced macrophage function, including increased inducible nitric oxide synthase expression (14). In contrast, the later stages of infection are characterized by a persistent fungal burden associated with decreased granuloma size and decreased inducible nitric oxide synthase expression. Chronically infected rats are asymptomatic and disease can be exacerbated by the administration of corticosteroids.
Macrophages are central to the effective immune response to C. neoformans in this model as well as in human infection. Besides direct antifungal activity, a variety of roles have been attributed to macrophages, including antigen presentation, polysaccharide sequestration, and cytokine and chemokine induction (13, 25, 33). Furthermore, there is evidence that persistent infection is associated with the intracellular residence of yeast cells in macrophages. The recognition of this encapsulated pathogen by macrophages generally requires opsonization via serum proteins like complement or antibody. It is therefore not surprising that many functions of macrophages in response to cryptococcal infection are described in association with phagocytosis of C. neoformans.
Monocyte chemoattractant protein (MCP-1) is a CC chemokine produced by a variety of cells that stimulates the recruitment of monocytes and T cells into areas of inflammation. MCP-1 is important in experimental models of granuloma formation (7). Furthermore, enhanced production of MCP-1 by alveolar macrophages (AM) from patients with sarcoidosis has been described (19). MCP-1 is produced during experimental murine cryptococcosis (27), and neutralization of MCP-1 results in decreased cell recruitment and increased fungal burden (17). Mice deficient in CCR-2, the receptor for MCP-1, exhibit deficient recruitment of monocytes in response to pulmonary cryptococcosis (31). Furthermore, the presence of both MCP-1 and CCR-2 are important in the polarization of the immune response towards a protective TH1 response during cryptococcal infection in mice (32). In previous studies, we showed that antibody-mediated phagocytosis of C. neoformans by human fetal microglia results in MIP-1α and MIP-1β and interleukin 8 RNA production. The process is in part dependent on Fc cross-linking (11). In the presence of sera, human monocytes but not alveolar macrophages produce MCP-1 in response to challenge with C. neoformans (23). Together these studies indicate that phagocytosis can be important for chemokine induction. These results also suggest that the type of opsonin and the activation state of monocytes/macrophages may regulate chemokine induction associated with C. neoformans-pulmonary macrophages (PuM) interactions. The purpose of this study then was to investigate production of MCP-1 within the lung during various stages of infection, including the role of PuM and phagocytosis in this process.
MATERIALS AND METHODS
Organism.
C. neoformans 24067, a serotype D strain, was obtained from the American Type Culture Collection (Manassas, Va.). Serotype D strains are pathogenic in humans and represent the majority of isolates in certain regions such as northern Europe. This strain was selected for study because it had been used in prior studies of cryptococcal pathogenesis in rats (9, 10). Organisms were grown in Sabouraud's dextrose broth (Difco, Detroit, Mich.) at 30°C, washed three times, suspended in sterile Hanks' balanced saline solution (HBSS), and counted in a hemacytometer. Inocula were confirmed by plating. For some experiments, C. neoformans was killed by heating to 70°C for 30 min.
Animals and intratracheal infection.
Male Fisher rats (Taconic Farms, Germantown, N.Y.) weighing 200 to 250 g, approximately 2.5 months old, were anesthetized by exposure to isoflurane and infected intratracheally with 5 × 106 organisms with an otoscope as described previously (10). Controls consisted of both noninfected rats (age, ∼2.5 months) and rats inoculated with sterile HBSS.
Lavage for PuM.
Rats were killed by asphyxiation with CO2, and the tracheas were cannulated with a 20-gauge angiocath (Becton Dickinson, Sandy, Utah) Lungs were lavaged 10 times with sterile HBSS without phenol red (Life Technologies, Grand Island, N.Y.) with 1 mM EGTA (Sigma). Lavage fluids were pooled, and cells were collected by centrifugation. Red blood cells were lysed by incubation in 0.17 M NH4Cl at 4°C for 10 min. Cells were then washed and suspended in Dulbecco's minimal essential medium (Life Technologies) with 10% heat-inactivated fetal calf serum (Bioproducts for Science, Indianapolis, Ind.). Approximately 2 × 106 PuM cells were obtained per rat. Cell viability was >95%, as measured by trypan blue vital dye exclusion. Staining with α-naphthyl acetate esterase (Sigma) showed that >95% of cells were of macrophage/monocyte/histiocyte lineage.
In vitro phagocytosis.
PuM were plated in 96-well tissue culture plates (Costar Corp., Cambridge, Mass.) at a density of 105 cells/ml in 0.1 ml of Dulbecco's minimal essential medium-10% fetal calf serum and allowed to attach at 37°C for 3 h. The media were then replaced with media (0.1 ml) containing 2 × 106 yeast cells/ml and either 10% rat serum or monoclonal antibody 18B7 (10 μg/ml). PuM and C. neoformans (effector-to-target cell ratio, 1:2) were incubated for 2 h at 37°C. Cells were gently washed with HBSS, fixed with ice-cold absolute methanol, and stained with Giemsa (Sigma). The phagocytic index was defined as the number of attached and ingested cryptococci divided by the number of macrophages per microscope field. Experiments were done in triplicate, and five different fields were counted for each well.
Opsonins.
18B7 is a murine immunoglobulin G1 (IgG1) monoclonal antibody that recognizes glucuronoxylomannan (GXM), the main component of the cryptococcal polysaccharide capsule (2). Serum was obtained from uninfected control rats, whose ages were approximately 2.5 to 3 months, and kept frozen at −20°C prior to use. All serum was used within 1 week of being obtained.
Inhibition studies.
Antibodies that recognize CD11b/c (OX-42; BD Pharmingen, San Diego, Calif.) and CD-18 (WT.3; Pharmingen) were added to PuM cultures during the attachment phase. After attachment, the media were removed and replaced with media containing C. neoformans in the presence of normal rat serum, as described for phagocytosis assays. Cultures were then used for phagocytosis or MCP-1 assays. Antibody concentrations of 5, 10, and 20 μg/ml were used with similar results. Irrelevant isotype-matched murine IgG1 and IgG2a (Pharmingen) at concentrations of 10 μg/ml were used as controls. To ensure that the addition of macrophage-reactive antibodies in the presence of serum did not result in macrophage lysis, cells were counted before and after antibody treatment. No differences in cell number were detected between cells treated with specific antibody, isotype-matched antibody, and media alone.
In vivo phagocytosis.
Rats were anesthetized with 70 mg of sodium pentobarbital (Anpro Pharmaceutical, Arcadia, Calif.)/kg of body weight, and their tracheae were surgically exposed and cannulated. Heat-killed C. neoformans, 107 in 0.3 ml of HBSS, was then injected intratracheally. Twenty-four hours following injection, PuM were removed by lung lavage and washed as described above. Cells were plated on 96-well tissue culture plates at a density of 106 cells/ml for 1 h at 37°C to allow for attachment. Extracellular organisms were gently washed off with HBSS, and cell monolayers were fixed with ice-cold absolute methanol and stained with Giemsa. Phagocytic indices were determined as described above. C. neoformans were not detected in the PuM cultures from infected rats injected intratracheally with HBSS alone.
Flow cytometry.
Cultured PuM (2 × 105) were preincubated for 15 min at 4°C with mouse gamma globulin (Pharmacia and Upjohn, Uppsala, Sweden) at a concentration of 500 μg/ml to block nonspecific Fc receptor binding. Cells were then incubated with fluorescein isothiocyanate-conjugated antibody specific for ED1 (a rat monocyte/macrophage marker) and phycoerythrin-conjugated antibodies specific for either CD11b/c (OX42; Serotec, Oxford, United Kingdom) or major histocompatibility complex Ia (MHC Ia) (OX3, Serotec). Cell were incubated with specific monoclonal antibodies for 30 min at 4°C. For intracellular staining, cells were consecutively fixed and permeabilized with Leukoperm reagent (Serotec) prior to incubation with specific antibody. After being washed extensively and fixed in 2% paraformaldehyde, cells were subjected to flow cytometric analysis by FACScan (Becton Dickinson) with CELLQuest software (Becton Dickinson). Individual populations of cells were gated according to scatter characteristics and ED1 staining. Controls for infection consisted of PuM harvested from rats at various times after intratracheal challenge with HBSS and nonmanipulated rats. For flow cytometry controls, isotype-matched irrelevant antibodies were used.
MCP-1 levels.
For MCP-1 lung homogenate level determinations, rats (5 per group) were killed at various lengths of time of infection. Lungs were finely minced in 5 ml of HBSS and further homogenized by treatment with a tissue terrator (Biospec Products, Racine, Wis.) for 2 cycles of 30 seconds each at the maximum setting. Lungs obtained from uninfected, nonmanipulated rats and HBSS-challenged rats were used as controls. Homogenates were centrifuged at 500 × g for 10 min to remove cell debris, and supernatants were frozen at −80°C. Supernatants were assayed for MCP-1 by using an Opt enzyme immunoassay enzyme-linked immunosorbent assay (ELISA) (BD Pharmingen) as per the manufacturer's instructions. For in vitro experiments, MCP-1 levels were determined in the supernatants of PuM obtained from various lengths of time of infection. For these experiments, PuM were incubated with heat-killed C. neoformans in the presence of 10% rat sera or specific antibody as described for phagocytosis experiments. Negative controls consisted of macrophages incubated with either sera, C. neoformans, or media alone. As a positive control, macrophages were stimulated with lipopolysaccharide from Escherichia coli 0127BB (100 ng/ml; Sigma) that was prepared by gel filtration. Following stimulation for 4 h, media was changed and cells were returned to the incubator. Supernatants were removed 24 h after initial stimulation, and MCP-1 levels were determined by ELISA.
MCP immunohistochemistry.
Rats were killed by asphyxiation with CO2, and lungs were inflated by intratracheal installation of optimal cutting temperature compound (OCT; Sakura Finetek USA, Torrance, Calif.). Lungs were removed and snap frozen in isopentane. Tissue sections (5 μm thick) were fixed with ice-cold methanol. Immunohistochemistry was performed as described with an indirect method. For MCP-1 staining, the primary antibody was a murine IgG1 anti-rat MCP-1 (Pharmingen) at a dilution of 1:100. For GXM and CD11b/c staining, primary antibodies were a murine IgG2a, 3E5, at a concentration of 10 μg/ml (3) and OX-42, which was also a murine IgG2a (Serotec). Secondary antibodies were alkaline phosphatase-labeled peroxidases. For double staining, individual stains were performed sequentially as described previously (9).
Statistical analysis.
For multiple comparisons between single groups, a one-way analysis of variance was done. For multiple comparisons between two groups, a two-way analysis of variance was done. For post hoc analysis, the data were compared by using the Dunnett's T3 test if the Levene test indicated significant nonhomogeneity of variance. Otherwise, the Tukey test was used. A P value of ≤0.05 was considered significant. Statistics were calculated using SPSS Base 10 (SPSS, Chicago, Ill.)
RESULTS
Phagocytic activity.
PuM from control (uninfected) rats exhibited enhanced phagocytic activity for C. neoformans in the presence of sera although the actual amount of phagocytosis was small (Fig. 1A). In contrast, phagocytosis was significantly more efficient with specific antibody. Serum-mediated phagocytosis was enhanced if fungal cells were incubated with serum prior to incubation with macrophages and if PuM were treated with granulocyte-macrophage colony-stimulating factor for 24 h prior to exposure to C. neoformans (data not shown).
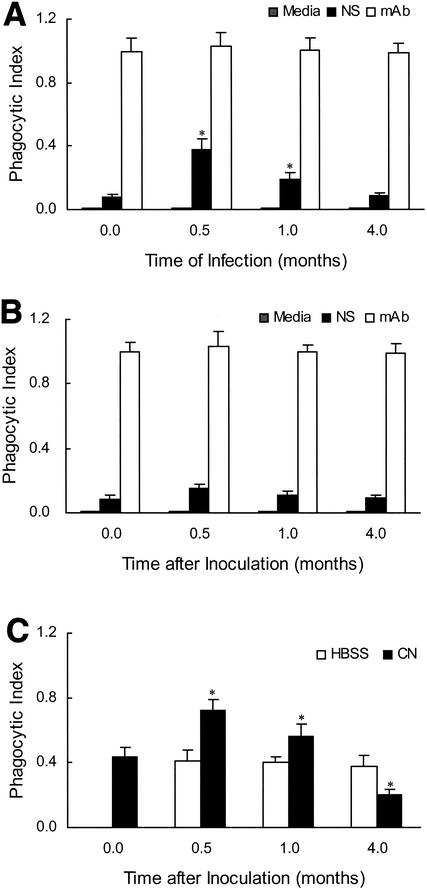
FIG. 1.
Phagocytic activity of PuM for C. neoformans varies over the course of infection. PuM were isolated from uninfected rats, rats at various lengths of time (0.5, 1, and 4 months) of infection (A), or HBSS-challenged rats (B). Cells were then exposed to C. neoformans in the presence of normal serum (NS) or specific antibody (mAb). The data shown represent the means and standard deviations for the combined results of several separate experiments. Six rats were used per length of time of infection, 6 uninfected control rats were used, and 9 rats were used for the HBSS challenge. *, P ≤ 0.05 compared to the phagocytic index of PuM from uninfected rats. (C) Phagocytic indices of PuM for C. neoformans with an in vivo assay. At various times following infection or HBSS challenge, rats were inoculated with heat-killed C. neoformans (CN). A lavage was done, and 24 h later, a phagocytic index was determined. Values represent the means and standard deviations obtained from the combination of several separate experiments in which a total of 6 rats per length time of infection was used. Instillation of HBSS was not associated with any changes in phagocytic index compared to nonmanipulated controls. *, P ≤ 0.05 compared to the phagocytic index of PuM from control rats.
The ability of PuM to affect phagocytosis of C. neoformans in the presence of serum varied over the course of infection (Fig. 1A). During the early stages of infection (0.5 to 1 months), the phagocytic activity of PuM was enhanced relative to nonmanipulated control and HBSS-challenged rats. By 4 months of infection, the phagocytic activity of PuM was decreased relative to shorter lengths of time of infection. When specific antibody was used as the opsonin, no temporal variation in the amount of phagocytosis was observed. Regardless of the length of time of infection when macrophages were harvested, specific antibody was more efficient in promoting phagocytosis than was serum. Intratracheal instillation of HBSS alone did not result in significant alterations in the phagocytic activity of PuM.
We also analyzed the phagocytic activity of PuM for C. neoformans with an in vivo assay. These experiments confirmed our in vitro experiments, showing variation in the phagocytic activity of PuM in association with infection. Enhanced phagocytosis was observed in the early stages (0.5 to 1 months) of infection when compared to macrophages from nonmanipulated control rats and HBSS-challenged rats. (Fig. 1C). In contrast, PuM from the later stages (4 months) of infection exhibited reduced phagocytosis of C. neoformans compared to macrophages from control uninfected rats. Intratracheal instillation of HBSS resulted in no alterations in phagocytic activity.
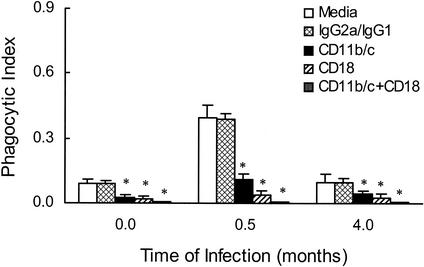
In additional in vitro experiments, incubation of PuM with either anti-CD11b/c or anti-CD18 resulted in a significant decrease in serum-mediated phagocytosis (Fig. 2). When both antibodies were used together, phagocytosis was inhibited >95% relative to the amount observed in the absence of antibody. In contrast, isotype-matched irrelevant antibody had no effect on the efficiency of complement-mediated phagocytosis. To determine whether the observed enhancement in phagocytosis during infection was related to an increase in complement receptor-mediated phagocytosis, we performed experiments with PuM from different lengths of time of infection (Fig. 2). The enhanced phagocytic activity of PuM was greatly decreased by treating PuM with antibodies to either CD11b/c or CD18.
FIG. 2.
Antibodies to CD11b/c and CD18 inhibit serum-mediated phagocytosis. PuM from infected rats, at various lengths of time of infection, were preincubated with media or antibodies specific for either CD11b/c or CD18 or both and then exposed to C. neoformans in the presence of normal serum. The data shown represent the means and standard deviations obtained from the combination of several separate experiments in which a total of 6 rats per length of time were studied. IgG2a and IgG1 are two irrelevant murine antibodies that were used in combination, each at a concentration of 10 μg/ml. *, P value of ≤0.05 compared to media-treated controls from the same length of time of infection.
CD11b/c and MHC expression.
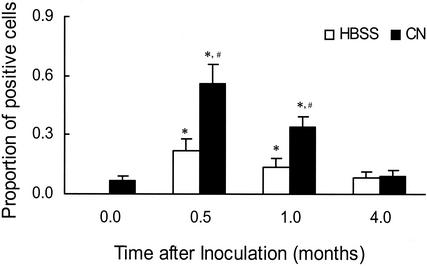
CD11b/c expression and its role in the efficiency of PuM phagocytic activity were determined by flow cytometry (Fig. 3). Expression of CD11b/c varied depending on the time of harvest, with the greatest expression at 0.5 months of infection, after which there was a progressive decrease in expression. By 4 months, no differences in receptor expression between infected and control rats could be detected. A similar time-related pattern was observed for MHC II expression (data not shown). A small but statistically significant increase in CD11b/c expression by PuM was observed both at 0.5 and 1 month after HBSS inoculation. PuM from both control rats and rats infected for 4 months exhibited enhanced CD11b/c expression following treatment with granulocyte-macrophage colony-stimulating factor (data not shown).
FIG. 3.
CD11b/c expression by PuM varies during pulmonary cryptococcosis. PuM were obtained from rats at various lengths of time of infection or HBSS challenge and subjected to flow cytometry. The data shown represent the mean values obtained from the combination of several separate experiments in which a total of 6 to 11 rats were used per length of time of infection or HBSS challenge. *, P value of ≤0.05 compared to macrophages from control nonmanipulated rats; #, P value of ≤0.05 compared to macrophages from HBSS-challenged rats; CN, C. neoformans.
In vivo MCP-1 levels.
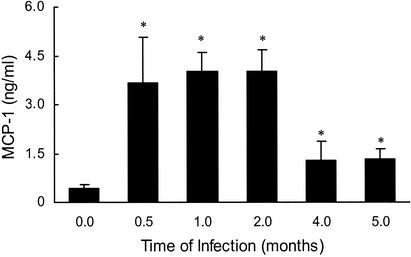
By 0.5 months after infection, increased MCP-1 levels were detected when compared to homogenates from nonmanipulated control rats (Fig. 4). Maximal levels of MCP-1 were present between 0.5 and 2 months of infection. MCP-1 levels subsequently decreased but remained elevated at 5 months of infection compared to those of nonmanipulated controls. In contrast, 1 month after inoculation with HBSS, levels of MCP-1 in the lung were not different from those of uninfected controls (data not shown).
FIG. 4.
Levels of MCP-1 in the lungs vary over the course of infection. Rats were infected with C. neoformans and killed at various lengths of time of infection. Lungs were removed and homogenized as described in Materials and Methods. MCP-1 levels were determined in supernatants by using a commercially available ELISA kit. The values shown represent the means from a representative experiment in which 5 rats per length of time of infection were studied. This experiment was repeated with similar results (data not shown). *, P value of ≤0.05 compared to MCP-1 levels in the supernatants of lungs obtained from uninfected controls.
MCP immunohistohemsitry.
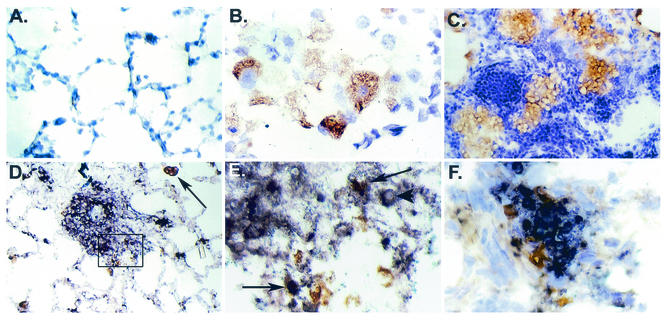
MCP-1 immunoreactivity was rarely seen in lung tissue from uninfected control rats (Fig. 5A). In contrast, immunohistochemical studies of lungs from rats at 1.5 months of infection revealed that MCP-1 reactivity was present primarily in PuM and epithelioid cells within granulomas (Fig. 5B and C). MCP-1-reactive macrophages were observed in affected areas of the lung as well as areas without any apparent involvement. MCP-1 reactivity was also occasionally observed in perivascular areas and endothelial cells (not shown). Double labeling for CD11b/c and MCP-1 confirmed that the cells producing MCP-1 were macrophages (Fig. 5D and E). Colocalization of GXM and MCP-1 in affected areas of the lung was also observed (Fig. 5F).
FIG. 5.
Immunohistochemistry for MCP-1. (A) Staining for MCP-1 in the lungs of control uninfected rats revealed minimal reactivity. Original magnification, ×313. (B) Staining for MCP-1 in rat lung on day 45 of infection reveals immunoreactivity within a cluster of alveolar macrophages. Original magnification, ×870. (C) MCP-1 reactivity is localized to epithelioid cells in organized granulomatous areas. Original magnification, ×313. (D) Double staining for MCP-1 (brown) and CD11b/c (blue) shows colocalization of MCP-1 in some macrophages and epithelioid cells. The black arrow points to an isolated macrophage that is stained for both CD11b/c and MCP-1. Original magnification, ×174. (E) Higher magnification (×870) of the boxed region in panel D. The black arrows point to cells that are double stained for MCP-1 (brown) and CD11b/c (blue). The arrowhead points to C. neoformans within a macrophage. (F) Double staining for MCP-1 (brown) and cryptococcal polysaccharide (blue) shows MCP-1-reactive cells surrounding the affected area. Original magnification, ×870.
In vitro MCP-1 levels.
Incubation of PuM with C. neoformans in the presence of sera resulted in MCP-1 induction (Fig. 6A). No induction was seen for cells treated with either sera or C. neoformans alone (data not shown). MCP-1 production in association with serum-mediated phagocytosis was lower than MCP-1 production in association with antibody-mediated phagocytosis. Incubation of PuM with antibodies against both CD11b/c and CD18 abolished the induction of MCP-1 observed following exposure to serum-opsonized C. neoformans (data not shown). This inhibition was not observed when irrelevant antibody was used.
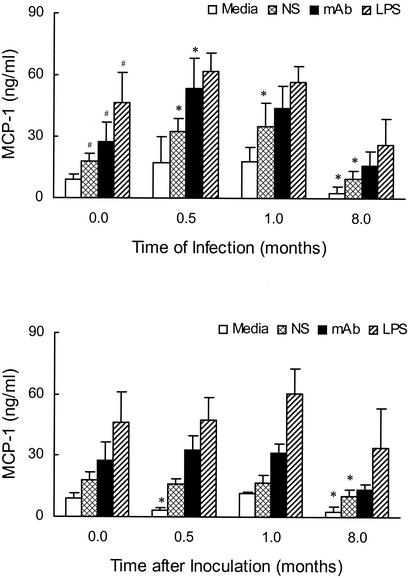
FIG. 6.
MCP-1 production by PuM following infection with C. neoformans. PuM were obtained from rats at various lengths of time of infection (A) or HBSS challenge (B). Cells were exposed to a variety of stimuli, including media alone, lipopolysaccharide (LPS), and C. neoformans in the presence of either serum (NS) or specific antibody (mAb). MCP-1 levels were determined by ELISA in the culture supernatant 24 h after initial stimulation. The values shown represent the means of several experiments for which a total of 6 to 9 rats were used per length of time studied. *, P ≤ 0.05 compared to similarly stimulated PuM from uninfected control rats. #, P ≤ 0.05 compared to nonstimulated PuM from uninfected control rats.
Regardless of stimuli, the amounts of MCP-1 produced by PuM from rats at 0.5 and 1 month of infection were greater than that produced by PuM from uninfected control rats (P values of 0.02 and 0.04, respectively). With respect to individual stimuli, significant increases in MCP-1 production were observed for PuM exposed to serum- and antibody-opsonized C. neoformans at 0.5 months of infection when compared to PuM from uninfected control rats. Enhanced MCP-1 production by PuM was not observed for HBSS-challenged rats (Fig. 6B). To study the effects of long-term infection, MCP-1 production by PuM from rats infected for 8 to 10 months and HBSS-matched controls was determined. PuM from both long-term-infected and HBSS-matched controls (average age, ∼11 months) produced less MCP-1 in the absence of stimulation and following exposure to serum-opsonized C. neoformans than did PuM from uninfected control rats (average age, ∼2 months). These results are consistent with the hypothesis that aging itself may impair MCP-1 production by PuM.
DISCUSSION
In previous studies, we showed that internalization of C. neoformans by alveolar macrophages precedes the influx of inflammatory cells and subsequent granuloma formation. These results suggest that C. neoformans-macrophage interactions are important in chemokine induction (10). Here we demonstrate that macrophages are an important source of MCP-1 in pulmonary C. neoformans infection and that phagocytosis can serve as a stimulus for MCP-1 induction. Since endotracheal challenge of rats with C. neoformans results in a persistent infection, our model enabled us to evaluate longitudinal changes in MCP-1 production during a process that is characterized by persistence and chronicity.
In this study, we show that serum-mediated phagocytosis of C. neoformans by rat PuM is dependent on the interaction of C. neoformans with CD11b/c and CD18 and establish that the efficiency of this process varies as much as fourfold over the course of infection. Maximal macrophage phagocytic activity was observed at 0.5 to 1 month of infection, which correlated with an increase in CD11b/c expression by PuM. Nevertheless, specific antibody was significantly more effective in promoting phagocytosis than serum at all stages of infection. In vivo phagocytosis experiments also revealed alterations in the phagocytic activity of pulmonary macrophages during infection. In these experiments, a defect in phagocytic activity was observed at 4 months of infection. The basis for this defect is unknown, but it was not observed in in vitro experiments, suggesting that something within the local environment may adversely effect phagocytosis. We have observed that GXM persists within the lung tissue during chronic infection, and we hypothesize that this could potentially have interfered with in vivo phagocytosis. Previous studies have shown that incubation of C. neoformans in normal sera results in extensive deposition of C3 on the capsule of C. neoformans which is rapidly converted to iC3b (22). iC3b can be bound by CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) on the surface of monocytes and macrophages. Our studies highlight the role of CR3 and CR4 expression in modulating complement-mediated phagocytosis in pulmonary cryptococcal infection and are consistent with those investigators who demonstrate the importance of CR3 and CR4 in serum-mediated phagocytosis of C. neoformans by human and murine macrophages (20, 24). Since dendritic cells are known to both phagocytize C. neoformans and express CD11c, it is possible that some of the observed variation in phagocytic activity during infection relates to changes within this population of cells (30).
Pulmonary challenge with C. neoformans resulted in enhanced MCP-1 production within the lung, with levels peaking between 0.5 and 2 months of infection. These findings are consistent with those from murine models of pulmonary cryptococcosis (17, 27) and illustrate the importance and conserved nature of MCP-1 induction in response to cryptococcal infection. Persistent but smaller elevations in MCP-1 levels were also present at 5 months of infection. These results are consistent with the notion that the regulation of MCP-1 expression may play a role in persistence. PuM from infected rats produced MCP-1 in a manner that paralleled the total lung MCP-1 levels. Immunohistochemical studies confirmed production of MCP-1 by PuM as well as granulomatous macrophages and epithelioid cells. Taken together, these results illustrate a role for macrophages in the production of MCP-1 within the lungs during cryptococcal infection.
Our studies further indicate that the interaction of C. neoformans and PuM via complement and antibody can result in MCP-1 induction at all stages of infection. Nevertheless, the enhanced production of MCP-1 by PuM from infected rats in the absence of exogenous stimuli suggests that there are likely to be other important signals for MCP-1 production during infection. These results are consistent with those of Chensue et al. who found that depletion of CD4+ T cells resulted in a significant reduction in MCP-1 production in a murine model of schistosomiasis (4).
While both complement- and antibody-mediated phagocytosis of C. neoformans resulted in MCP-1 induction, antibody-mediated phagocytosis consistently resulted in greater MCP-1 production. The basis for the discrepancy remains to be determined, but it could be related to quantitative differences in phagocytosis or differences in signal transduction associated with these different phagocytic pathways. These findings may help explain some of the beneficial effects of specific antibody therapy in animal models of cryptococcosis and the observation that administration of specific antibody enhances granuloma formation (6).
Levels of MCP-1 in the lungs were decreased during the later stages of infection. The signals responsible for down-modulating MCP-1 production during the later stages of infection remain to be determined. Down modulation of various elements of the immune response have been described previously in association with cryptococcal infection, including antibody responsiveness (16), lymphoproliferative responses (28), and macrophage activation (26). Interestingly, we found that MCP-1 production by PuM from older rats was diminished in comparison to the response of PuM from younger rats. This was true for both spontaneous MCP-1 production (i.e., nonstimulated cells) and MCP-1 induction associated with serum-mediated phagocytosis. Previous studies have demonstrated an enhanced susceptibility of aged mice to C. neoformans infection (1). Impairment of a variety of alveolar macrophage functions in association with aging has been described previously, including nitric oxide production (21), accessory cell function (34), and cytokine production (18). Recently, Corsini et al. found that the defect in tumor necrosis factor alpha production by macrophages from senescent rats reflects an impairment in the protein kinase C pathway (5). Interestingly, we have found that MIP-1α induction associated with antibody-mediated phagocytosis of C. neoformans in human microglia involves activation of the mitogen-activated protein kinase pathway, which may be activated by the protein kinase C pathway (29). The findings in this study are consistent with the hypothesis that the age-associated changes in chemokine production result from defective signaling.
In summary, our study demonstrates a role for macrophages in the production of MCP-1 during pulmonary cryptococcosis. We further demonstrate that phagocytosis of C. neoformans mediated by either antibody or complement can serve as an important stimulus for MCP-1 induction, although antibody-mediated phagocytosis is a more-potent stimulus for MCP-1 production. Our findings also suggest that down-regulation of chemokine production by macrophages may play a role in promoting persistence and that old age may result in defective chemokine production by PuM.
Acknowledgments
A.C. is supported by NIH grants AI33774, AI3342, and HL-59842-01 and is a recipient of a Burroughs Wellcome Development Therapeutics Award. D.L.G. is supported by NIH grant AIO1300.
We thank Ramata Niang for excellent help in tissue processing.
Editor: T. R. Kozel
REFERENCES
- 1.Aguirre, K. M., G. W. Gibson, and L. L. Johnson. 1998. Decreased resistance to primary intravenous Cryptococcus neoformans infection in aged mice despite adequate resistance to intravenous rechallenge. Infect. Immun. 66:4018-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendevai, J. Mukherjee, L.-A. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadevall, A., J. Mukherjee, S. J. N. Devi, R. Schneerson, J. B. Robbins, and M. D. Scharff. 1992. Antibodies elicited by a Cryptococcus neoformans glucuronoxylomannan-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J. Infect. Dis. 65:1086-1093. [DOI] [PubMed] [Google Scholar]
- 4.Chensue, S. W., K. S. Warmington, N. W. Lukacs, P. M. Lincoln, M. D. Burdick, R. M. Strieter, and S. L. Kunkel. 1995. Monocyte chemotactic protein expression during schistosome egg granuloma formation. Am. J. Pathol. 146:130-138. [PMC free article] [PubMed] [Google Scholar]
- 5.Corsini, E., F. Battaini, L. Lucchi, M. Marinovich, M. Racchi, S. Govoni, and C. L. Galli. 2002. A defective protein kinase C anchoring system underlying age-associated impairment in TNF-α production in rat macrophages. J. Immunol. 163:3468-3473. [PubMed] [Google Scholar]
- 6.Feldmesser, M., and A. Casadevall. 1997. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J. Immunol. 158:790-799. [PubMed] [Google Scholar]
- 7.Flory, C. M., M. L. Jones, and J. S. Warren. 1993. Pulmonary granuloma formation in the rat is partially dependent on monocyte chemoattractant protein 1. Lab. Investig. 69:396-404. [PubMed] [Google Scholar]
- 8.Garcia-Hermoso, D., G. Janbon, and F. Dromer. 1999. Epidemiological evidence for dormant Cryptococcus neoformans infection. J. Clin. Microbiol. 37:3204-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman, D., Y. Cho, M.-L. Zhao, A. Casadevall, and S. C. Lee. 1996. Expression of inducible nitric oxide synthase in rat pulmonary Cryptococcus neoformans granulomas. Am. J. Pathol. 148:1275-1282. [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman, D., S. C. Lee, and A. Casadevall. 1994. Pathogenesis of pulmonary Cryptococcus neoformans infection in the rat. Infect. Immun. 62:4755-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman, D., X. Song, R. Kitai, A. Casadevall, M.-L. Zhao, and S. C. Lee. 2001. Cryptococcus neoformans induces macrophage inflammatory protein 1α (MIP-1α) and MIP-1β in human microglia: role of specific antibody and soluble capsular polysaccharide. Infect. Immun. 69:1808-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldman, D. L., H. Khine, J. Abadi, D. J. Lindenberg, L.-A. Pirofski, R. Niang, and A. Casadevall. 2001. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107:E66.. [DOI] [PubMed] [Google Scholar]
- 13.Goldman, D. L., S. C. Lee, and A. Casadevall. 1995. Tissue localization of Cryptococcus neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infect. Immun. 63:3448-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman, D. L., S. C. Lee, A. J. Mednick, L. Montella, and A. Casadevall. 2000. Persistent Cryptococcus neoformans pulmonary infection in the rat is associated with intracellular parasitism, decreased inducible nitric oxide synthase expression and altered antibody responsiveness to cryptococcal polysaccharide. Infect. Immun. 68:832-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haugen, R. K., and R. D. Baker. 1954. The pulmonary lesions in cryptococcosis with special reference to subpleural nodules. Am. J. Clin. Pathol. 24:1381-1390. [DOI] [PubMed] [Google Scholar]
- 16.Henderson, D. K., J. E. Bennett, and M. A. Huber. 1982. Long-lasting, specific immunologic unresponsiveness associated with cryptococcal meningitis. J. Clin. Investig. 69:1185-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huffnagle, G. B., R. M. Strieter, T. J. Standiford, R. A. McDonald, M. D. Burdick, S. L. Kunkel, and G. B. Toews. 1995. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J. Immunol. 155:4790-4797. [PubMed] [Google Scholar]
- 18.Inamizu, T., M. P. Chang, and T. Makinodan. 1985. Influence of age on the production and regulation of IL-1 in mice. Immunology 55:447.. [PMC free article] [PubMed] [Google Scholar]
- 19.Iyonaga, K., M. Suga, H. Ichiyasu, T. Yamamoto, Y. Hiraga, and M. Ando. 1998. Measurement of serum monocytic chemoattractant protein-1 and clinical applications for estimating the activity of granuloma formation in sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 15:165-172. [PubMed] [Google Scholar]
- 20.Kawakami, K., K. Shibuya, M. H. Qureshi, T. Zhang, Y. Koguchi, M. Tohyama, Q. Xie, S. Naoe, and A. Saito. 1999. Chemokine responses and accumulation of inflammatory cells in the lungs of mice infected with highly virulent Cryptococcus neoformans: effects of interleukin-12. FEMS Immunol. Med. Microbiol. 25:391-402. [DOI] [PubMed] [Google Scholar]
- 21.Koike, E., T. Kobayashi, M. Katsumi, and M. Murakami. 1999. Effect of aging on nitric oxide production by rat alveolar macrophages. Exp. Gerontol. 34:889-894. [DOI] [PubMed] [Google Scholar]
- 22.Kozel, T. R., and G. S. T. Pfrommer. 1986. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect. Immun. 52:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levitz, S. M., E. A. North, Y. Jiang, S. H. Nong, and H. Kornfeld. 1997. Variables affecting production of monocyte chemotactic factor 1 from human leukocytes stimulated with Cryptococcus neoformans. Infect. Immun. 65:903-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levitz, S. M., and A. Tabuni. 1991. Binding of Cryptococcus neoformans by human cultured macrophages. Requirements for multiple complement receptors and actin. J. Clin. Investig. 87:528-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levitz, S. M., A. Tabuni, H. Kornfeld, C. C. Reardon, and D. T. Golenbock. 1994. Production of tumor necrosis factor alpha in human leukocytes stimulated by Cryptococcus neoformans. Infect. Immun. 62:1975-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masih, D. T., C. E. Sobomayer, L. A. Cervi, C. M. Riera, and H. R. Rubinstein. 1991. Inhibition of I-A expression in rat peritoneal macrophages due to T-suppressor cells induced by Cryptococcus neoformans. J. Med. Vet. Mycol. 29:125-128. [DOI] [PubMed] [Google Scholar]
- 27.Olszewski, M. A., G. B. Huffnagle, T. R. Traynor, R. A. McDonald, D. N. Cook, and G. B. Toews. 2001. Regulatory effects of macrophage inflammatory protein 1α/CCL3 on the development of immunity to Cryptococcus neoformans depend on the expression of early inflammatory cytokines. Infect. Immun. 69:6256-6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson, B. E., N. K. Hall, G. S. Bulmer, and R. Blackstock. 1982. Suppression of responses to cryptococcal antigen in murine cryptococcosis. Mycopathologia 80:157-163. [DOI] [PubMed] [Google Scholar]
- 29.Song, X., S. Shapiro, D. L. Goldman, A. Casadevall, M. D. Scharff, and S. C. Lee. 2002. Fcγ receptor I- and III-mediated macrophage inflammatory protein 1α induction in primary human and murine microglia. Infect. Immun. 70:5177-5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syme, R. M., J. C. L. Spurrell, E. K. Amankwah, F. H. Y. Green, and C. H. Mody. 2002. Primary dendritic cells phagocytose Cryptococcus neoformans via mannose receptors and Fcγ receptor II for presentation to T lymphocytes. Infect. Immun. 70:5972-5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traynor, T. R., A. C. Herring, M. E. Dorf, W. A. Kuziel, G. B. Toews, and G. B. Huffnagle. 2002. Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J. Immunol. 168:4659-4666. [DOI] [PubMed] [Google Scholar]
- 32.Traynor, T. R., W. A. Kuziel, G. B. Toews, and G. B. Huffnagle. 2000. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J. Immunol. 164:2021-2027. [DOI] [PubMed] [Google Scholar]
- 33.Vecchiarelli, A., M. Dottorini, D. Pietrella, C. Monari, C. Retini, T. Todisco, and F. Bistoni. 1994. Role of human alveolar macrophages as antigen-presentation cells in Cryptococcus neoformans infection. Am. J. Respir. Cell Mol. Biol. 11:130-137. [DOI] [PubMed] [Google Scholar]
- 34.Zissel, G., M. Schlaak, and J. Muller-Quernheim. 1999. Age-related decrease in accessory cell function of human alveolar macrophages. J. Investig. Med. 47:51-56. [PubMed] [Google Scholar]