Abstract
Mycobacterium ulcerans is the causative agent of Buruli ulcer, a severe necrotizing skin disease endemic in tropical countries. Clinical evidence suggests that M. ulcerans isolates from Asia, Mexico, and Australia may be less virulent than isolates from Africa. In vivo studies suggest that mycolactone, a polyketide-derived macrolide toxin, plays a major role in the tissue destruction and immune suppression which occur in cases of Buruli ulcer. Mycolactones were extracted from 34 isolates of M. ulcerans representing strains from Africa, Malaysia, Asia, Australia, and Mexico. Thin-layer chromatography, mass spectroscopic analysis, and cytopathic assays of partially purified mycolactones from these isolates revealed that M. ulcerans produces a heterogeneous mixture of mycolactone variants. Mycolactone A/B, the most biologically active mycolactone species, was identified by mass spectroscopy as [M+Na]+ at m/z 765.5 in all cytotoxic isolates except for those from Mexico. Mycolactone C [M+Na]+ at m/z 726.3 was the dominant mycolactone species in eight Australian isolates, and mycolactone D [M+Na]+ m/z 781.2 was characteristic of two Asian strains. Mycolactone species are conserved within specific geographic areas, suggesting that there may be a correlation between mycolactone profile and virulence. In addition, the core lactone, [M+Na]+ m/z 447.4, was identified as a minor species, supporting the hypothesis that mycolactones are synthesized by two polyketide synthases. A cytopathic assay of the core lactone showed that this molecule is sufficient for cytotoxicity, although it is much less potent than the complete mycolactone.
Mycobacterium ulcerans is the causative agent of Buruli ulcer, a severe ulcerative skin lesion which has major foci of endemicity in Australia and western Africa (16). Although M. ulcerans is closely related to Mycobacterium tuberculosis, the organism produces an extracellular rather than an intracellular infection in humans (12). Buruli ulcer is characterized by extensive tissue damage, which occurs in the absence of an acute inflammatory response. There is considerable variation in the clinical presentations of Buruli ulcer patients, and evidence suggests that there are geographical differences in the severity and forms of the disease (16). The first sign of infection with M. ulcerans is usually a hard subcutaneous nodule. As the disease develops, massive edema may occur in the subcutaneous tissue. In most cases, a large open ulcer forms several weeks or even months after the initial infection. In some cases, however, and particularly in Benin, a plaque form of the disease develops prior to ulceration. Although the disease process is usually limited to subcutaneous tissue, osteomyelitis has been documented. All of these forms of the disease, including the nodular, edematous, plaque, and ulcerative forms and osteomyelitis, are found among western African patients. However, there also appears to be geographical variation in the pathology of the disease (16). For example, the plaque form of Buruli ulcer, though common in Benin, appears to be entirely absent in Australia. Osteomyelitis, which is fairly common in Benin and Ghana, has not been reported from Australia or Mexico, suggesting that the M. ulcerans isolates from outside western Africa may be less virulent. Other work suggests that infections with Asian isolates may also be less virulent than those with the African strains (6).
The major virulence determinant in M. ulcerans is a polyketide-derived macrolide, mycolactone. Mycolactone was originally isolated from M. ulcerans 1615, a Malaysian isolate, as a mixture of cis/trans isomers designated mycolactone A and mycolactone B. Identical molecules were also found to be present in two M. ulcerans isolates from the Democratic Republic of Congo (11). More-recent evidence shows that M. ulcerans 1615 produces a family of mycolactone congeners which differ primarily in the number of hydroxyl groups and double bonds (2).
Mycolactone appears to play a key role in the pathogenesis of Buruli ulcer. In vivo studies using a guinea pig model of infection suggest that mycolactone is responsible for both the extensive tissue damage and immunosuppression which accompanies Buruli ulcer (9). The activity of mycolactone on cultured fibroblasts and macrophage cell lines produces a distinct cytopathic phenotype. The earliest effect is cell rounding, which occurs within 10 h after addition of mycolactone to cultured cells. At 36 h, treated cells are arrested in G1 of the cell cycle, and by 72 h, cells begin to die via apoptosis (10).
Bacterial macrolides are produced as secondary metabolites by soil bacteria, particularly bacteria such as Streptomyces and Saccharopolyspora species in the order Actinomycetales (14). Interestingly, a number of related macrolides or congeners are often produced by a single bacterial strain (17). Cadapan et al. have recently shown that M. ulcerans 1615 produces several minor species of mycolactone (congeners) in addition to mycolactone A/B (2). The purpose of this study was to determine whether all strains of M. ulcerans produce mycolactones, whether geographical variants of mycolactone exist, whether these differ in potency, and whether a correlation can be established between production of a particular macrolide and a specific form of M. ulcerans disease.
In this work we have isolated and partially characterized mycolactones from 34 clinical isolates of M. ulcerans representing patient isolates from Benin, the Democratic Republic of Congo, Australia, Mexico, Japan, and China. Strains from Benin include isolates from patients representing the full clinical spectrum of M. ulcerans disease. Whereas almost all of the isolates tested produce mycolactone A/B, there is considerable heterogeneity in the mycolactone congeners produced by strains from different geographical areas. These molecules differ in potency, although the biological activity is conserved. Despite the various forms of clinical disease represented by the Benin strains, there was no correlation between the amount or form of mycolactone produced and the clinical form of the disease. Finally, analysis of minor mycolactone species led to the discovery that one of the polyketide precursors to mycolactone can be isolated from the bacterial surface as an intact active molecule, supporting the hypothesis that mycolactone is synthesized by two separate polyketide synthases.
MATERIALS AND METHODS
Bacterial strains and culture.
All strains were low-passage clinical isolates which had been maintained at −80°C (Table 1). Isolates were received in our laboratory on Lowerstein-Jensen slants and were transferred to Middlebrook 7H9 medium supplemented with 10% oleic acid-albumin-dextrose enrichment (OADC; Difco)-2% glycerol. Strains were grown at 31°C in 25-cm2 tissue culture flasks (Falcon) and then transferred at a 1:200 dilution to 200 ml in 150-cm2 tissue culture flasks. Mycolactone production is optimal under these growth conditions, though doubling time is greater than 72 h. Sterile filtrate (SF) from the culture was tested weekly for cytopathicity. Bacteria and culture filtrate were harvested at between 6 and 10 weeks, when cultures reached the late logarithmic growth phase and mycolactone production was at peak concentration.
TABLE 1.
Strain table
| Strain | Clinical feature | Yr of isolation | Location of isolation | Source or reference |
|---|---|---|---|---|
| 1615 | Ulcer | 1964 | Malaysia | 15 |
| PORT | Ulcer | 1969 | Democratic Republic of Congo | ITMa |
| 97-101 | Nodule | 1997 | Benin | ITM |
| 97-112 | Nodule | 1997 | Benin | ITM |
| 97-617 | Nodule | 1997 | Benin | ITM |
| 97-1112 | Nodule | 1997 | Benin | ITM |
| 97-110 | Nodule | 1997 | Benin | ITM |
| 98-700 | Edema | 1998 | Benin | ITM |
| 98-1137 | Edema | 1998 | Benin | ITM |
| 99-1563 | Edema | 1999 | Benin | ITM |
| 99-1711 | Edema | 119 | Benin | ITM |
| 97-616 | Plaque | 1997 | Benin | ITM |
| 97-1116 | Plaque | 1997 | Benin | ITM |
| 98-156 | Plaque | 1998 | Benin | ITM |
| 98-239 | Plaque | 1998 | Benin | ITM |
| 99-742 | Osteomyelitis | 1999 | Benin | ITM |
| 99-684 | Osteomyelitis | 1999 | Benin | ITM |
| 99-1642 | Osteomyelitis | 1999 | Benin | ITM |
| 99-1722 | Osteomyelitis | 1999 | Benin | ITM |
| 99-1567 | Osteomyelitis | 1999 | Benin | ITM |
| 94-1324 | Ulcer | 1994 | Australia | ITM |
| 94-1325 | Ulcer | 1994 | Australia | ITM |
| 94-1326 | Ulcer | 1994 | Australia | ITM |
| 94-1327 | Ulcer | 1994 | Australia | ITM |
| 94-1328 | Ulcer | 1994 | Australia | ITM |
| TS-1 | Ulcer | 1999 | Victoria, Australia | Stinearb |
| TS-2 | Ulcer | 1978 | Queensland, Australia | Stinear |
| TS-3 | Ulcer | 1999 | Victoria, Australia | Stinear |
| TS-4 | Ulcer | 1999 | Queensland, Australia | Stinear |
| TS-6 | Ulcer | 1999 | Queensland, Australia | Stinear |
| 5114 | Ulcer | 1953 | Mexico | ITM |
| 5143 | Ulcer | 1967 | Mexico | ITM |
| 98-912 | Ulcer | 1997 | China | ITM |
| 8756 | Ulcer | 1980 | Japan | ITM |
ITM, Institute of Tropical Medicine, B-2000 Antwerp, Belgium.
Stinear, Tim Stinear, Victorian Infectious Diseases Reference Laboratory, Melbourne, Victoria, Australia.
Isolation of mycolactone from bacteria and culture filtrate.
Mycolactone was partially purified as previously described (8). Briefly, bacterial cells were filtered through a 0.2-micron-pore-size filter, air dried, and weighed. Lipids were extracted from the bacterial pellet with chloroform-methanol (2:1, vol/vol) and dried in a rotoevaporator (Buchi) at 56°C. The resulting polar lipids were dissolved in a minimal volume of ice-cold acetone to precipitate the unwanted phospholipids and to enrich for mycolactones. In a similar manner, acetone-soluble lipids (ASLs) were prepared from culture filtrate. ASL preparations were analyzed by silica thin-layer chromatography (TLC) using chloroform-methanol-water (90:10:1, vol/vol/vol) as a solvent system. Lipids were visualized by oxidative charring with ceric sulfate-ammonium molybdate in 2 M sulfuric acid stain. Mycolactone A/B was identified as a major UV-active, light yellow lipid species with a refractive index of 0.23. Samples were further analyzed by electrospray ionization mass spectroscopy (MS) for the presence of a major peak at 765 (Na+) which represented mycolactone A/B. Where cytopathic activity (CPA) and physical evidence suggested the presence of additional mycolactone-related molecules, individual lipids were extracted from TLC plates, tested for cytopathicity, and analyzed by MS.
Cytopathic assays.
L929 murine fibroblasts were maintained in the lab in Dulbecco's modified Eagle's medium with 5% fetal calf serum in tissue culture flasks and incubated in 5% CO2 at 37°C as previously described (8). ASL samples or individual lipid species were dissolved in acetone, diluted in tissue culture medium, and added to cells in a 96-well tissue culture plate to determine cytopathicity. CPA was defined as the minimal concentration of ASLs per milliliter necessary to produce 90% cell rounding in 24 h and loss of the monolayer by 48 h.
MS analysis of M. ulcerans extracts.
Potential mycolactone-containing lipid extracts prepared previously were dissolved in ethanol to a final concentration of 10 μg/ml. The samples were then directly perfused into an electrospray ionization source on a Bruker Esquire 2000 mass spectrometer by using a Cole Palmer 74900 series syringe pump. The electrospray MS conditions were first optimized to a pure mycolactone standard, and these conditions were then applied to the lipid extracts. The electrospray MS conditions were as follows: infusion rate, 1,000 μl/h; nebulizer pressure, 30 lb/in2; dry gas flow, 10 liters/min, and dry temperature, 320°C; capillary voltage, −4,000 V; endplate offset, −500 V. The mycolactone production within each ASL sample was determined by the presence or absence of ions characteristic of the presence of mycolactone: the molecular ion (MH+[m/z743.5]), the more abundant sodium adduct [M+Na]+ (m/z765.5), and the dehydrated molecular ion M+-H2O (m/z725.5).
Base hydrolysis of mycolactone.
Mycolactone A/B (1 mg) was added to 0.5 ml of anhydrous methanol in which 3 to 4 mg of anhydrous K2CO3 had been previously dissolved.
This mixture was stirred at room temperature for 30 h. Mycolactone hydrolysis products were removed with a glass pipette and analyzed by silica TLC using chloroform-methanol-water (90:10:1, vol/vol/vol). The resulting products were eluted from TLC plates with chloroform-methanol (90:10, vol/vol), subjected to chemical analysis, and assayed for cytopathicity.
RESULTS
Biochemical analysis of mycolactones from M. ulcerans bacteria.
Mycolactones were partially purified by obtaining ASLs from M. ulcerans isolates when cultures reached the late logarithmic growth stage and macrolide production was at its peak. Some isolates grew more slowly than others, and the densities at which different isolates reached the late logarithmic phase differed considerably. This is reflected in the growth yields as well as in the yields of ASLs/100 mg of dry cell weight obtained (Table 2). All isolates of M. ulcerans produced large amounts of cell-associated lipids known as phthiocerol derivatives (4). This heterogeneous group of lipids appears as a large smear of nonpolar lipids in TLC experiments run in chloroform-methanol-water (90:10:1, vol/vol/vol) (Fig. 1A). George et al. have previously shown that M. ulcerans phthiocerols lack cytotoxic activity either for cultured cells or for animals (9). Mycolactone A/B was the second most abundant lipid species detected in M. ulcerans ASLs (Fig. 1A). All isolates of M. ulcerans, except 97-1112 (Benin), 94-1325 (Australia), and 5114 and 5143 (Mexico), produced mycolactone A/B as a major lipid species. Because the growth yield of isolate 97-1112 was extremely low, ASLs were isolated from an expanded 1-liter culture of this strain. Mycolactone A/B could be clearly detected in isolate 97-1112 when ASLs from this culture were analyzed (data not shown), suggesting that the previous negative results were due to inadequate cell mass (CM). Thus, mycolactone A/B was identified in all African isolates tested. In addition to mycolactone A/B, M. ulcerans 1615, as well as all African isolates, contained four minor cell-associated lipids which appear on TLC as a “lipid ladder” (Rfs 0.25, 0.32, 0.38, and 0.45) with decreasing polarity above mycolactone (Fig. 1A). This lipid ladder has been previously described for M. ulcerans 1615, a Malaysian isolate (9). Recent data show that these bands contain mycolactone congeners (2).
TABLE 2.
| Strain | Clinical feature | CM (mg) | Yield (mg) of ASLs from:
|
||
|---|---|---|---|---|---|
| Total CM | 100 mg of CM | 200 ml of SF | |||
| 1615 | Ulcer | 107 | 8.5 | 7.9 | 2 |
| PORT | Ulcer | 120 | 3.8 | 3.2 | 0.9 |
| 97-101 | Nodule | 127 | 3.6 | 2.8 | 2.3 |
| 97-112 | Nodule | 167 | 6.2 | 3.7 | 1.5 |
| 97-617 | Nodule | 13 | 4.2 | 32.3 | 0.9 |
| 97-1112 | Nodule | 33 | 3.0 | 9.1 | 1.2 |
| 97-110 | Edema | 123 | 3.4 | 2.8 | 1.7 |
| 98-700 | Edema | 108 | 3.6 | 3.3 | 1.5 |
| 98-1137 | Edema | 21 | 3.0 | 14.3 | 3.4 |
| 99-1563 | Edema | 42 | 3.0 | 7.1 | 2.0 |
| 99-1711 | Edema | 33 | 1.4 | 4.2 | 0.6 |
| 97-616 | Plaque | 121 | 4.0 | 3.3 | 2.0 |
| 97-1116 | Plaque | 304 | 5.5 | 1.8 | 1.3 |
| 98-156 | Plaque | 549 | 7.9 | 1.4 | 1.5 |
| 98-239 | Plaque | 78 | 1.8 | 2.3 | 7.3 |
| 99-742 | Osteomyelitis | 67 | 5.1 | 7.6 | 7.2 |
| 99-684 | Osteomyelitis | 175 | 4.0 | 2.3 | 0.9 |
| 99-1642 | Osteomyelitis | 156 | 4.6 | 2.9 | 1.1 |
| 99-1722 | Osteomyelitis | 182 | 1.5 | 0.8 | 1.8 |
| 99-1567 | Osteomyelitis | 109 | 3.6 | 3.3 | 1.9 |
| 94-1324 | Ulcer | 10 | 0.7 | 7.0 | 5.2 |
| 94-1325 | Ulcer | 239 | 4.0 | 1.7 | 5.0 |
| 94-1326 | Ulcer | 535 | 4.2 | 0.8 | 0.7 |
| 94-1327 | Ulcer | 708 | 5.5 | 0.8 | 0.7 |
| 94-1328 | Ulcer | 373 | 2.4 | 0.6 | 1.8 |
| 5114 | Ulcer | 256 | 3.5 | 1.4 | 1.3 |
| 5143 | Ulcer | 215 | 3.4 | 1.6 | 2.2 |
| 98-912 | Ulcer | 31 | 1.4 | 4.5 | 0.6 |
| 8756 | Ulcer | 90 | 2.1 | 2.3 | 3.4 |
ASLs of a chloroform-methanol (2:1, vol/vol) extract.
CM, cell mass extracted by filtration on 0.4-μm-pore-size filter and air dried.
ASLs extracted at late logarithmic growth phase.
FIG. 1.
Silica TLC of ASLs from Mycobacterium ulcerans isolates run in chloroform-methanol-water (90:10:1, vol/vol/vol) and visualized by oxidative charring in a ceric molybdate-10% sulfuric acid stain. (A) ASLs extracted from the bacterial CM. (B) ASLs extracted from sterile culture filtrate. MLA/B, mycolactone A/B; Rf, refractive indices; lane M, ASLs extracted from Middlebrook 7H9 media with 10% oleic acid-albumin-dextrose enrichment. Strains are represented in lanes as follows: lane 1, 97-617; lane 2, 97-112; lane 3, 97-101; lane 4, 97-1112; lane 5, 97-110; lane 6, 98-700; lane 7, 98-1137; lane 8, 99-1563; lane 9, 99-1711; lane 10, 97-1116; lane 11, 98-239; lane 12, 97-616; lane 13, 98-156; lane 14, 99-742; lane 15, 97-684; lane 16, 99-1642; lane 17, 99-1722; lane 18, 99-1567; lane 19, 94-1324; lane 20, 94-1325; lane 21, 94-1326; lane 22, 94-1327; lane 23, 94-1328; lane 24, 5114; lane 25, 5143; lane 26, 98-912; lane 27, 8756; lane 28, 1615.
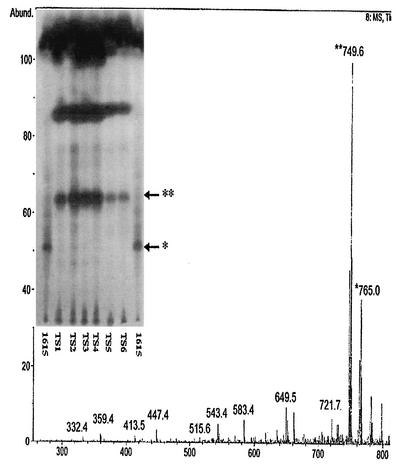
ASLs extracted from Australian isolates of M. ulcerans had a unique mycolactone profile. Whereas mycolactone A/B occurred as a major component of the ASLs from African isolates, mycolactone A/B was present in only trace amounts in ASLs extracted from Australian isolates. However, the Australian isolates contained two abundant lipids (Rf 0.38 and 0.65) which were considerably less polar than mycolactone A/B (Fig. 2). The single Japanese and Chinese isolates (Fig. 1A, lanes 26 and 27) had identical ASL profiles and appeared to contain mycolactone A/B along with a slightly more polar lipid (Rf 0.19) that did not appear as a major species in the African isolates. This species may correspond to a mycolactone congener detected by MS at [M+Na]+ m/z 781.3. If so, it represents a congener with an additional oxygen molecule. Finally, the two Mexican isolates (Fig. 1A, lanes 24 and 25) shared an identical lipid profile and contained a lipid species slightly more polar than mycolactone A/B (Rf 0.18).
FIG. 2.
Comparison of ASLs extracted from six Australian isolates of M. ulcerans (TS-1 to TS-6) with ASLs extracted from M. ulcerans 1615. Silica TLC was run in chloroform-methanol-water (90:10:1, vol/vol/vol) and visualized by oxidative charring in a ceric molybdate-10% sulfuric acid stain (insert). Mass spectroscopic analysis of ASLs from TS-2, showing the presence of mycolactone C as a major peak at m/z 749.6 along with the presence of mycolactone A/B at m/z 765.0, is shown. ∗, mycolactone A/B; ∗∗, mycolactone C, core lactone; Abund., % abundance.
In summary, TLC analysis of ASLs from M. ulcerans suggests that strains from similar geographic areas shared similar ASL patterns. Results from African strains collected over a span of 30 years suggest that mycolactone production is a stable strain characteristic. The same conservation of macrolide profile was found among Australian strains (eight isolates) collected over a span of 21 years and two Mexican strains whose dates of isolation were separated by 14 years. Although only two Asian isolates were analyzed, these two also shared a distinct ASL profile despite the fact that one was from China and the other from Japan.
MS analysis of ASLs extracted from bacterial pellets confirmed results from TLC.
ASLs from all African isolates contained a major peak at m/z 765.5 [M+Na]+, confirming the presence of mycolactone A/B. In addition, several minor peaks were detected in African isolates which are likely to represent mycolactone-related species. These include peaks at m/z 447.3, 726.5, 747.5, 749.5, and 781.3. The 747 and 749 species have recently been identified as mycolactone congeners in M. ulcerans 1615, although neither had been tested for biological activity (2). In the Australian species, the major ion detected in cell-associated ASLs was represented by a peak at m/z 749.5 (molecular weight [MW], 723.5). Assuming that this ion is a sodium adduct, this species corresponds to mycolactone A/B lacking oxygen. In this work we have shown that this congener is biologically active, and we have designated it mycolactone C (see Table 5). In addition to mycolactone C, Australian isolates also contained a minor mycolactone A/B peak at m/z 765.5. Both Asian isolates contained a major peak at m/z 781.3, designated mycolactone D, as well as a minor peak at m/z 765.5. The lipid detected at m/z 781.3 could be a newly discovered mycolactone congener containing an additional oxygen molecule. We were unable to identify mycolactone A/B, C, or D in ASLs from either of the Mexican strains, despite the fact that ASLs from both strains showed typical mycolactone cytopathicity on L929 cells (Table 3 and Table 4).
TABLE 5.
CPA of mycolactones
| Mycolactone | MW | CPA (ng/ml) |
|---|---|---|
| Mycolactone A/B | 743 | 0.01 |
| Mycolactone C | 726 | 800 |
| Core mycolactone | 424 | 12,500 |
TABLE 3.
Mycolactones identified in ASLs isolated from geographically diverse strains
| Source (no. of strains tested) | MW (major species)a | MW (minor species) |
|---|---|---|
| Malaysia (1) | 743b | 424c 724d 726e 759 |
| Western Africa (21) | 743 | 447, 723, 726, 759 |
| Australia (8) | 726 | 743 |
| Japan (1) | 758f | 743, 424 |
| China (1) | 758 | 743 |
MW based on MS detection of [M+Na]+.
Mycolactone A/B (11 strains).
Mycolactone core (1 strain).
Mycolactone congeners (2 strains).
Mycolactone C.
Mycolactone D.
TABLE 4.
CPAa of ASLs from CM and SF
| Strain | Clinical features (source) | CPA of ASLs (ng/ml)
|
CPA (ng/ml/100 mg of CM) | |
|---|---|---|---|---|
| CM | SF | |||
| 1615 | Ulcer (Malaysia) | 0.01 | 0.03 | 0.0093 |
| 97-617 | Nodule (Benin) | 2.56 | 608 | 19.69 |
| 97-112 | Nodule (Benin) | 320 | 189.44 | 191.61 |
| 97-101 | Nodule (Benin) | 64 | 58.62 | 50.39 |
| 97-1112 | Nodule (Benin) | Negb | Neg | |
| 94-110 | Edema (Benin) | 320 | 1,107.20 | 260.16 |
| 98-700 | Edema (Benin) | 1,600 | 966.40 | 1,481.48 |
| 98-1137 | Edema (Benin) | 1,600 | 2,176 | 7,619.04 |
| 99-1563 | Edema (Benin) | 320 | 1,276 | 761.90 |
| 99-1711 | Edema (Benin) | 64 | 396.80 | 193.93 |
| 97-1116 | Plaque (Benin) | 0.00654 | 85.12 | 0.0021 |
| 98-239 | Plaque (Benin) | 64 | 334.40 | 82.05 |
| 97-616 | Plaque (Benin) | 320 | 1,299.20 | 264.64 |
| 98-156 | Plaque (Benin) | 0.10 | 960 | 0.0182 |
| 99-742 | Osteomyelitis (Benin) | 1,600 | 458.88 | 2,388.05 |
| 99-684 | Osteomyelitis (Benin) | 1,600 | 576 | 1,019.10 |
| 99-1642 | Osteomyelitis (Benin) | 320 | 704 | 205.13 |
| 99-1722 | Osteomyelitis (Benin) | 12.80 | 230.40 | 7.03 |
| 99-1567 | Osteomyelitis (Benin) | 0.10 | 245.76 | 0.0917 |
| 94-1324 | Ulcer (Australia) | 1,056 | 8,400 | 10,560.00 |
| 94-1325 | Ulcer (Australia) | Neg | Neg | |
| 94-1326 | Ulcer (Australia) | 2.56 | 2,496 | 0.4785 |
| 94-1327 | Ulcer (Australia) | 64 | 2,080 | 9.04 |
| 94-1328 | Ulcer (Australia) | 12.8 | 232.96 | 3.43 |
| 5114 | Ulcer (Mexico) | 1,000 | 20,160 | 3,90.62 |
| 5143 | Ulcer (Mexico) | 5,000 | 6,912 | 2,325.58 |
| 98-912 | Ulcer (China) | 200 | 19,040 | 645.16 |
| 8756 | Ulcer (Japan) | 10.66 | 2,150 | 11.84 |
CPA is defined as the minimal concentration of ASL necessary to produce 90% cell rounding in 24 h and detached cells in 48 h.
Neg, negative results in cytopathic assays.
Thus, mycolactone A/B was identified as a cell-associated lipid in all isolates of M. ulcerans except for the two isolates from Mexico. In addition, both the TLC and MS results suggest the presence of a family of mycolactone-related molecules, and this heterogeneity correlates well with the geographic origins of the isolates (Table 3).
Isolation of mycolactone A/B from SF.
The presence of pathology distant from the site of bacterial colonization in Buruli ulcer provided the first clue that M. ulcerans made a secreted toxin (3). Mycolactone was initially identified in the SF of M. ulcerans culture and was later shown to be cell associated (8). One hypothesis for the differential virulence of M. ulcerans isolates from different geographical locations is that strains differ in their ability to secrete mycolactone. ASLs were extracted from 200 ml of M. ulcerans SF, analyzed by TLC, and tested for cytopathicity (Fig. 1B and Table 4). We had previously determined that in cultures grown without agitation, 75% of mycolactone was cell associated whereas 25% was able to be recovered from the culture filtrate. ASLs extracted from SF are less complex than those extracted from intact bacteria because of the absence of abundant phthiocerol derivatives in SF. TLC analysis of SF showed the presence of small amounts of mycolactone A/B in all M. ulcerans isolates tested except Benin isolate 97-1112, Australian isolate 94-1325, and the two Mexican isolates (Fig. 1B). A trace amount of mycolactone A/B was detected in the single Chinese isolate 98-912. In addition to Chinese isolate 98-912, Japanese isolate 8756 contained trace amounts of a slightly more polar lipid species with an Rf value of 0.21. In all strains except for those from Australia, the presence of cell-associated mycolactone was associated with a secreted form. Surprisingly, although bacterial ASLs from Australian isolates lacked a major mycolactone band, mycolactone A/B was clearly present in the SF. In contrast, the cell-associated mycolactones (Rf 0.38 and 0.65) were not detected in the SF from Australian isolates. MS analysis of ASLs from SF was not conducted, but cytopathic assays were performed on all SF ASLs to confirm the presence of mycolactones.
CPA of ASLs from M. ulcerans cells and SF.
Mycolactone has a very specific cytotoxic phenotype, which includes cell rounding (at 10 h), cell cycle arrest (36 h), and apoptotic cell death (72 h). George et al. have shown previously that all CPA found in ASLs is accounted for by the presence of mycolactones (9). As expected, there was correspondence between CPA and the presence of mycolactone as confirmed by TLC and MS (Fig. 1 and Table 4). In general, higher CPA was associated with bacterial ASLs compared to SF ASLs within a single isolate, reflecting the greater partitioning of mycolactones to the cell surface (Table 4). Many mycolactone congeners are absent from SF or, if present, occur in quantities below the level of detection. When cell-associated CPA is expressed as CPA per 100 mg of CM, it is clear that there is enormous strain variation in cell-associated CPA. The most cytopathic ASLs are extracted from African isolates but there are also the greatest number of African strains represented. Three out of four isolates with a CPA less than 100 ng/100 mg of CM were from Africa. Based on a very small sample size, cell-associated ASLs from Mexican and Asian isolates are generally less cytopathic than ASLs from other M. ulcerans isolates (Table 4).
It is not possible to directly compare SF CPA between strains from these data. This is because SF ASLs were isolated from a fixed volume of culture filtrate (200 ml from all isolates), whereas the growth yields from these cultures differed considerably.
One of the Australian isolates, 94-1325, appears not to produce mycolactone A/B. Consistent with this, ASLs extracted from both the bacteria and the SF lacked CPA, suggesting that this strain is a mycolactone-negative mutant.
Differences in mycolactone levels do not correlate with clinical forms of disease in Benin.
There did not appear to be a correlation between clinical presentation of disease and ASL profiles. Although M. ulcerans isolates from Benin were obtained from patients with nodular, edematous, plaque, and osteomyelitic forms of Buruli ulcer, ASL profiles did not differ markedly among these groups (Fig. 1 and Table 4). All isolates produced the typical lipid ladder of mycolactones described above for M. ulcerans 1615. Despite the fact that some strains grew better in vitro than others and that the better-growing strains appeared to produce more mycolactones, greater production of mycolactones did not correlate with a particular form of the disease.
Isolation and characterization of mycolactone-related molecules from geographically diverse strains of M. ulcerans.
Mycolactone is a polyketide-derived macrolide whose synthesis is likely to require the participation of two type 1 polyketide synthases. Type 1 polyketide synthases are very large enzymes containing multiple functional domains. Among macrolide-producing soil bacteria, it is extremely common for one bacterial strain to make multiple forms of a single macrolide. For example, some strains of Streptomyces venezuela make four related macrolides and Saccharopolyspora erythraea makes five forms of erythromycin (13, 17).
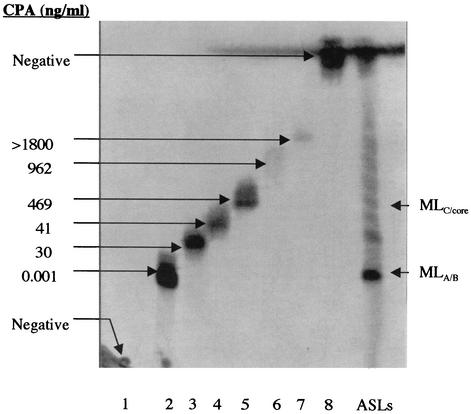
Examination of ASLs by TLC, MS, and cytopathicity assays suggested that M. ulcerans strains might produce a family of mycolactones and, furthermore, that the particular repertoire of mycolactones produced might be a strain characteristic. Cadapan et al. have shown biochemical evidence that M. ulcerans 1615 makes several mycolactone congeners (2); however, none of these were tested for biological activity. To assess the activity of these minor mycolactone species, individual lipid bands were eluted from TLC plates and assessed for cytopathicity. Significant problems were encountered in attempting to purify these minor mycolactones to homogeneity, making it impossible to obtain structural details for these molecules. First, they are produced in tiny amounts by the bacteria; second, extensive isomerization occurs at double bonds in mycolactone upon exposure to light, making the molecule instable. Nonetheless, we were able to establish that in M. ulcerans 1615, mycolactone A/B is both the most abundant and most cytopathic lipid among the mycolactones and that CPA tends to decrease with decreasing polarity (Fig. 3). This evidence is consistent with previous work showing that hydroxyl groups were required for activity (9).
FIG. 3.
Cytopathicity of individual lipid species in ASLs from M. ulcerans 1615. Individual lipids from bands 1 to 8 were isolated using preparative silica TLC run in a chloroform-methanol-water (90:10:1, vol/vol/vol) solvent system. For cytopathicity assays, twofold dilutions of individual lipids from bands 1 to 8 were added to L929 fibroblasts and scored for CPA. CPA is defined as the least amount of lipid (in nanograms per milliliter) required to cause 90% cell rounding in 24 h and monolayer detachment after 48 h. Lane ASLs, ASLs extracted from M. ulcerans 1615 with chloroform-methanol (2:1, vol/vol); MLA/B, mycolactone A/B; MLC/core, mix of mycolactone C and core lactone.
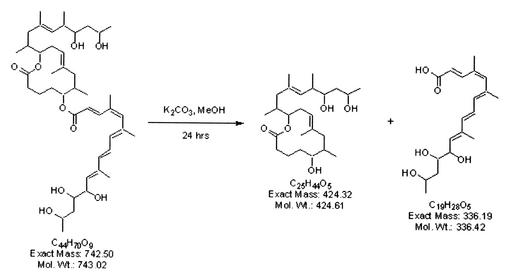
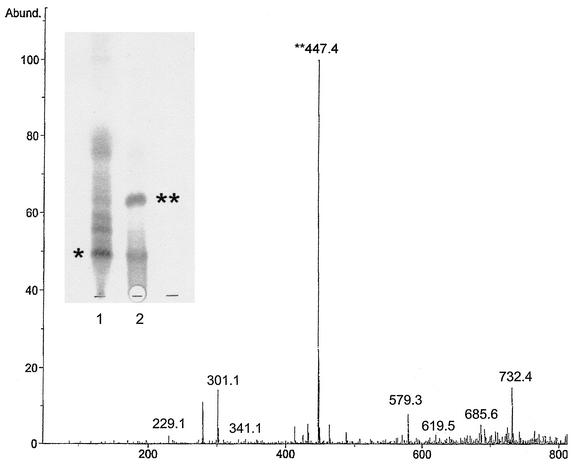
It was possible to obtain structural information for one of the cytopathic lipids in the lipid ladder. Evidence from TLC of M. ulcerans 1615 ASLs suggested that the major lipid species in band 5 (Fig. 3) represent the core lactone (Fig. 4). Although it was not possible to obtain sufficient pure material for complete structural determination of the major lipid in band 5, proton NMR showed the presence of a major lipid species which was deficient in conjugated double bonds. This finding was consistent with a loss of the conjugated double bonds which are a prominent feature of the fatty acid side chain. Mass spectroscopic analysis of this lipid revealed a major [M+Na]+ at m/z 447.4, a finding consistent with the presence of the core lactone. To confirm this, mycolactone A/B was subjected to base hydrolysis (Fig. 4) and the products were isolated using TLC (Fig. 5, insert). MS revealed that the less polar of the hydrolysis products contained the expected ion at m/z 447.4, corresponding to the core lactone (Fig. 5). When the free lactone obtained from hydrolysis of mycolactone was compared with free lactone synthesized by the Kishi laboratory (1), they were found to be identical. When the purified core lactone obtained by hydrolysis was tested for cytopathicity, it was surprising to discover that this molecule produced a cytopathic phenotype that is nearly identical to that of mycolactone A/B, although the core lactone was 10,000-fold less active than mycolactone (Table 5).
FIG. 4.
Base hydrolysis of mycolactone. Mycolactone (left) was stirred with potassium carbonate in anhydrous methanol for 24 h at room temperature. Hydrolysis products include the core lactone and fatty acid side chain.
FIG. 5.
Mass spectroscopic analysis of the upper band containing the core lactone product. (Insert) Results of silica TLC, showing products of base hydrolysis of mycolactone and hydrolysis products, are shown. Lane 1, mycolactone starting material; lane 2, hydrolysis products; ∗, mycolactone; ∗∗, core lactone; Abund., % abundance.
Similar efforts were made to purify mycolactone variants from isolates obtained from Australia, Asia, and Mexico. Analysis of individual lipids from Australian isolates (94-1327, TS-1, and TS-2) showed that CPA was associated with a light yellow, UV-active lipid which appeared on TLC with a refractive index of 0.38. Trace amounts of mycolactone A/B were also detected at Rf 0.23 (Fig. 2). Mass spectroscopic analysis of the most abundant active lipid, mycolactone C, revealed a major peak at m/z 749.5 (Fig. 2, insert). This is consistent with loss of oxygen from mycolactone A/B and the reduction in polarity observed by TLC (Fig. 1A and 2). Mass spectroscopic analysis revealed trace amounts of mycolactone A/B. When tested on L929 cells, mycolactone C appeared to be significantly less active than mycolactone A/B (Table 5). As previously mentioned, mycolactone C occurs as a minor mycolactone in ASLs from M. ulcerans 1615 (2) as well as in isolates from Benin (data not shown).
In a similar manner, attempts were made to correlate CPA with a specific lipid species in the Japanese strain 8756. ASLs from strain 8756 were separated by TLC. Individual lipids were eluted from TLC, tested for cytopathicity, and analyzed by MS. CPA was associated with a light yellow, UV-active lipid which produced a peak at m/z 781. This lipid was designated mycolactone D. Assuming that this is a sodium adduct of mycolactone, an ion detected at 781.3 would be consistent with the addition of an oxygen molecule to mycolactone A/B. Although mycolactone D produced typical mycolactone activity on L929 cells, sufficient material to provide accurate quantitative data was lacking.
We were unable to assign CPA to a specific molecular species in ASLs extracted from Mexican isolates. Although cytopathic lipids were eluted from TLC of Mexican ASLs, mass spectroscopic analysis of the eluted lipids showed the presence of numerous lipid species. Further, as mentioned previously, ASLs from the two Mexican isolates did not contain mycolactone A/B or any of the other newly identified mycolactones.
In summary, unique patterns of mycolactone production are characteristic of M. ulcerans strains isolated from specific geographic areas (Table 3). Although it was not possible to fully characterize all of these mycolactone congeners, data suggest that these unique patterns persist through time.
DISCUSSION
Evidence presented in this paper shows that M. ulcerans strains produce a family of related macrolides with identical biological activities but differing potencies. The specific repertoire of mycolactone congeners produced by strains shows geographical clustering. Mycolactone A/B is the most potent of the M. ulcerans macrolides and, with the exception of the Mexican isolates, all M. ulcerans produce mycolactone A/B. African strains, along with the single Malaysian isolate M. ulcerans 1615, produce a greater amount and variety of mycolactones than strains from Mexico, Asia, or Australia. In African isolates and M. ulcerans 1615, mycolactone A/B is produced as a major lipid species along with at least four additional mycolactone congeners. Since all mycolactones are cytopathic, the presence of multiple mycolactone congeners in these strains contributes to their greater cytopathicity. Preliminary evidence suggests that M. ulcerans isolates from Asia and Mexico have unique mycolactone profiles, although many more isolates must be tested to confirm this. It is particularly interesting that neither of the two Mexican strains produced detectable amounts of mycolactone A/B, C, or D. Nonetheless, the fact that SF and ASLs from these strains produced typical mycolactone CPA is strong evidence that a species of mycolactone is present.
M. ulcerans 1615 is a particularly interesting isolate. This strain was obtained from the Trudeau collection, where it is described as a Malaysian isolate cultured in 1964 from an Aboriginal patient (15). It might be expected that the mycolactone profile of this strain would resemble that of Australian strains. However, the mycolactone profile of 1615 is identical to that of African strains. M. ulcerans 1615 has been recently typed by Tim Stinear at the Pasteur Institute. Taxonomically, the strain appears to fall somewhere between the African and Malaysian clusters (personal communication).
In western Africa, nodular, edematous, plaque, ulcerative and osteomyelitis forms of Buruli ulcer are all present (16). One of the goals of this investigation was to determine whether there was an association between the clinical form of M. ulcerans and mycolactone production. However, we found no evidence that there was an association between the clinical form of the disease and the number of mycolactones produced by an isolate.
Clinical evidence also suggests that Buruli ulcer cases from different geographic areas differ in severity. For example, M. ulcerans osteomyelitis, a more invasive form of the disease, has not been described outside of Africa. Chinese isolates of M. ulcerans (subspecies shinshuense) are reportedly considerably less virulent than those from Africa (6). There may be a correlation between severity of disease and mycolactone profile, since African strains, which produce the greatest number and quantity of mycolactones, are associated with more-severe disease. It is important to point out, however, that many other factors, such as host biology, economics, cultural views on disease, and availability of health care facilities, play a significant role in determining the severity of Buruli ulcers. Because of this, it is unlikely that differences in mycolactone production are sufficient to account for the observed geographical differences in the severity of Buruli ulcer.
The first cases of Buruli ulcer were described from central Africa in 1897, although the first African isolates were not cultured until 1964 (5). More recently, the focus of endemicity of M. ulcerans infection appears to have shifted from central to western Africa. Despite the intervening decades, strains from western and central Africa share identical mycolactone profiles, suggesting a common origin for these strains. Isolates obtained from Australia include strains from Victoria and Queensland—locations a thousand miles apart. Despite this finding, the mycolactone profile of Australian isolates appears to have been conserved over a period of 21 years. Thus, mycolactone profiles appear to be highly conserved within a geographic area.
The findings presented in this report provide some insight into the biosynthesis of the mycolactones. Mycolactone A/B is composed of two polyketide chains, one of which undergoes spontaneous cyclization to form the core lactone, a 12-member lactone ring (7). The genetics inferred from this structure suggest that at least three enzymes participate in the biosynthesis of mycolactone—two polyketide synthases, to form the two polyketide chains, and an acyl transferase, to combine the two chains. The isolation of the core mycolactone [M+Na]+ at m/z 447 (MW, 424) from the cell surface provides the first evidence for the existence of a precursor molecule. This evidence fits well with the proposed model for mycolactone synthesis. A surprising finding was that the core lactone is sufficient for cytopathicity. The enormous difference in potency between the core lactone and the complete mycolactone molecule suggests that the function of the fatty acid side chain is that of expediting entry of the molecule into the cell or of enabling it to efficiently interact with an intracellular target molecule.
What is the mechanism by which a single isolate of M. ulcerans produces a heterogeneous mixture of mycolactones? Among macrolide-producing soil bacteria, the production of numerous macrolide congeners is a common theme. For example, a single isolate of Saccharopolyspora erythraea produces five erythromycin congeners (13), whereas Streptomyces venezuela (17) produces four related antibiotics. From analogy with these examples, there are two possible basic mechanisms by which M. ulcerans produces mycolactone congeners. One possibility is that a heterogeneous mixture of mycolactones is produced by differential β-keto processing on a type I polyketide synthase. Alternatively, some strains may have specific p450 hydroxylases which modify the polyketide backbone, thus providing distinctive patterns of hydroxylation. We are in the process of analyzing a set of mycolactone-negative mutants recently constructed in the laboratory. Mapping these mutants suggests that over 100 kb of DNA is dedicated to the biosynthesis of this molecule. This work, along with the completion of the M. ulcerans genome project being undertaken by Stewart Cole and Tim Stinear at the Pasteur Institute, should provide a window into understanding polyketide synthesis in mycobacteria species. Nonetheless, the fact that M. ulcerans has a doubling time greater than 36 h suggests that real progress in genetic analysis requires expression of mycolactone in a fast-growing heterologous host.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Armand Mve-Obiang was supported by a grant from the World Health Organization.
We acknowledge the excellent graphic support of Brian Ranger. Purified core lactone and technical advice were graciously provided by Andrew Benowitz and Yoshito Kishi, Department of Chemistry and Chemical Biology, Harvard University. We thank Diane Welty (Rocky Mountain Laboratories, NIAID, NIH) for outstanding technical assistance.
Editor: J. T. Barbieri
REFERENCES
- 1.Benowitz, A. B., S. Fidanze, P. L. Small, and Y. Kishi. 2001. Stereochemistry of the core structure of the mycolactones. J. Am. Chem. Soc. 123:5128-5129. [DOI] [PubMed] [Google Scholar]
- 2.Cadapan, L. D., R. L. Arslanian, J. R. Carney, S. M. Zavala, P. L. Small, and P. Licari. 2001. Suspension cultivation of Mycobacterium ulcerans for the production of mycolactones. FEMS Microbiol. Lett. 205:385-389. [DOI] [PubMed] [Google Scholar]
- 3.Connor, D. H., and H. F. Lunn. 1965. Mycobacterium ulcerans infection. Int. J. Lepr. 33:698-709. [PubMed] [Google Scholar]
- 4.Daffé, M. D., A. Varnerot, and V. V. Levy-Frebault. 1992. The phenolic mycoside of Mycobacterium ulcerans: structure and taxonomic implications. J. Gen. Microbiol. 138:131-137. [DOI] [PubMed] [Google Scholar]
- 5.Dodge, O. G. 1964. Mycobacterial skin ulcers in Uganda; histopathological and experimental aspects. J. Pathol. Bacteriol. 88:167-174. [DOI] [PubMed] [Google Scholar]
- 6.Faber, W. R., L. M. Arias-Bouda, J. E. Zeegalaar, A. H. Kolk, P. A. Fonteyne, J. Toonstra, and F. Portaels. 2000. First reported case of Mycobacterium ulcerans infection in a patient from China. Trans. R. Soc. Trop. Med. Hyg. 94:277-279. [DOI] [PubMed] [Google Scholar]
- 7.Fidanze, S., F. Song, M. Szlosek-Pinaud, P. L. Small, and Y. Kishi. 2001. Complete structure of the mycolactones. J. Am. Chem. Soc. 123:10117-10118. [DOI] [PubMed] [Google Scholar]
- 8.George, K. M., L. Barker, D. Welty, and P. L. C. Small. 1998. Partial purification and characterization of biological effects of a lipid toxin produced by Mycobacterium ulcerans. Infect. Immun. 66:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George, K. M., D. Chatterjee, G. Gunawardana, D. M. Welty, J. Hayman, R. Lee, and P. L. C. Small. 1999. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283:854-857. [DOI] [PubMed] [Google Scholar]
- 10.George, K. M., L. Pascopella, D. M. Welty, and P. L. C. Small. 2000. A Mycobacterium ulcerans toxin, mycolactone, causes apoptosis in guinea pig ulcers and tissue culture cells. Infect. Immun. 68:877-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunawardana, G., D. Chatterjee, K. M. George, P. Brennan, D. Whittern, and P. L. C. Small. 1999. Characterization of novel macrolide toxins, mycolactones A and B from a human pathogen, Mycobacterium ulcerans. J. Am. Chem. Soc. 121:6092-6093. [Google Scholar]
- 12.Hayman, J. 1993. Out of Africa: observations on the histopathology of Mycobacterium ulcerans infection. J. Clin. Pathol. 46:5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz, L. 1997. Manipulation of modular polyketide synthases. Chem. Rev. 97:2557-2576. [DOI] [PubMed] [Google Scholar]
- 14.Katz, L., and S. Donadio. 1993. Polyketide synthesis: prospects for hybrid antibiotics. Annu. Rev. Microbiol. 47:875-912. [DOI] [PubMed] [Google Scholar]
- 15.Pettit, J. H. S., N. J. Marchette, and R. J. W. Rees. 1966. Mycobacterium ulcerans infection. Clinical and bacteriological study of the first cases recognized in South East Asia. Br. J. Dermatol. 78:187-197. [DOI] [PubMed] [Google Scholar]
- 16.van der Werf, T. S., W. T. van der Graaf, J. W. Tappero, and K. Asiedu. 1999. Mycobacterium ulcerans infection. Lancet 354:1013-1018. [DOI] [PubMed] [Google Scholar]
- 17.Xue, Y., L. Zhao, H.-W. Liu, and D. H. Sherman. 1998. A gene cluster for macrolide antibiotic biosynthesis in Streptomyces venezuelae: architecture of metabolic diversity. Proc. Natl. Acad. Sci. USA 95:12111-12116. [DOI] [PMC free article] [PubMed] [Google Scholar]