Abstract
This study investigates Toll-like receptor 4 (TLR4)-positive macrophages in early recognition and clearance of pulmonary bacteria. TLR4 is a trans-membrane receptor that is the primary recognition molecule for lipopolysaccharide of gram-negative bacteria. The TLR4Lps-del mouse strains C57BL10/ScN (B10) and STOCK Abbtm1 TLR4Lps-del Slc11a1s(B10 × C2D) are susceptible to pulmonary infections and develop pneumonia when naturally or experimentally infected by the opportunistic bacterium Pasteurella pneumotropica. Since these mice have the TLR4Lps-del genotype, we hypothesized that reconstitution of mice with TLR4-positive macrophages would provide resistance to this bacterium. A cultured macrophage cell line (C2D macrophages) and bone marrow cells from C2D mice were adoptively transferred to B10 and B10 × C2D mice by intraperitoneal injection. C2D macrophages increased B10 and B10 × C2D mouse resistance to P. pneumotropica. In C2D-recipient mice there was earlier transcription of tumor necrosis factor alpha and chemokines JE and macrophage inflammatory protein 2 (MIP-2) in the lungs of B10 and B10 × C2D mice, and there was earlier transcription of KC and MIP-1α in B10 × C2D mice. In addition, the course of inflammation following experimental Pasteurella challenge was altered in C2D recipients. C2D macrophages also protected B10 × C2D mice, which lack CD4+ T cells. These data indicate that macrophages are critical for pulmonary immunity and can provide host resistance to P. pneumotropica. This study indicates that TLR4-positive macrophages are important for early recognition and clearance of pulmonary bacterial infections.
Through the course of normal gas exchange, the lung is continuously exposed to a variety of potential infectious agents (21). Due to the physical characteristics of the lung, it is an ideal environment for microbial colonization and growth. Both innate and acquired host defense mechanisms help prevent pulmonary infection (13, 30).
Alveolar macrophages are the first cells to encounter foreign microbes and particulates within the alveolus (11, 31). Alveolar macrophages can detect and destroy potential pathogens, recognize antigens, and produce proinflammatory cytokines to obviate microbial infection (8). Alveolar macrophages use proteins encoded in the germ line to recognize a wide variety of microbial molecules. One of these proteins is Toll-like receptor 4 (TLR4). TLR4 is a trans-membrane receptor that is the primary recognition molecule of the macrophage in response to lipopolysaccharide (LPS) of gram-negative bacteria (4-6, 27). TLR4 is a pattern recognition receptor that recognizes conserved portions of microbial molecules termed pathogen-associated molecular patterns (17). The pattern recognition receptor-pathogen-associated molecular pattern interaction of TLR4 with LPS activates macrophages through the TLR4 signal transduction pathway and leads to NF-κB activation and the production of proinflammatory mediators (8).
Impaired immunity can lead to pulmonary infections by opportunistic bacteria. The TLR4Lps-del mouse strains C57BL10/ScN (B10) and STOCK Abbtm1 TLR4Lps-del Slc11a1s (B10 × C2D) are both susceptible to infection by the opportunistic gram-negative bacterium Pasteurella pneumotropica. These mice develop severe, and sometimes lethal, pneumonia when experimentally challenged with P. pneumotropica (9). In contrast, the major histocompatibility complex class II-null (MHCII−/−) and TLR4Lps-n mouse strain, C2D, is largely resistant in spite of the absence of CD4+ T cells. We have achieved long-term reconstitution of TLR4Lps-del mice with either TLR4-positive whole bone marrow or a macrophage cell line, C2D. (M. L Hart et al., submitted for publication). Adoptively transferred cells colonize areas that characteristically contain macrophages, including the lungs (33). Because the TLR4 gene controls macrophage activation and is necessary for protection against P. pneumotropica-induced pneumonia, we hypothesized that reconstitution of TLR4Lps-del mice with TLR4-positive macrophages would provide protection against this bacterium. This study shows that macrophages are the critical component necessary for resistance to experimental P. pneumotropica infection in mice.
MATERIALS AND METHODS
Mouse strains.
To determine the impact of MHCII genes, the C57BL/6J (B6 MHCII+/+ TLR4Lps-n) mouse strain, originally obtained from the Jackson Laboratory (Bar Harbor, Maine), was routinely used for congenic comparisons to C2D mice (15, 32) (Table 1). C2D mice (B6.129 Abbtm1 N5F20 MHCII−/− TLR4Lps-del) lack functional MHCII genes due to a natural deletion of the IEα gene and the targeted deletion of the IAβ gene (15, 16). C2D mice were backcrossed five times to the B6 background, and the offspring of such are considered an incipient congenic strain (Jackson Laboratory notes, 2000). The C2D mouse has been brother-sister mated for more than 20 generations for 9 years. C57BL10/ScN (B10 MHCII+/+ TLR4Lps-del) mice were obtained from the animal resource facility at the National Institutes of Health, bred in the mouse facility at Kansas State University since 1995, and used as founders for the B10 × C2D recombinant stock. The B10 × C2D mice (STOCK Abbtm1 TLR4Lps-del Slc11a1s), created at Kansas State University were previously described (9, 34). Breeder mice were routinely genotyped for quality assurance. All mouse experiments were approved by the Institutional Animal Care and Use Committee at Kansas State University.
TABLE 1.
Mouse strains used in this study
| Mouse strain | Abbreviation | Genotype |
|---|---|---|
| B6.129 Abbtml | C2D | MHCII−/−TLR4Lps-n/Lps-n |
| C57BL10/ScN | B10 | MHCII+/+TLR4Lps-del/Lps-del |
| STOCK Abbtml TLR4Lps-del Slc11a1s | B10 × C2D | MHCII−/−TLR4Lps-del/Lps-del |
Genotyping. (i) MHCII.
Mice were genotyped by assaying for the disrupted MHCII Aβ gene fragment using reverse transcription (RT)-PCR of mRNA obtained from peripheral white blood cells. Total RNA was prepared from white blood cells using TRIzol-LS (Invitrogen, Carlsbad, Calif.) according to the manufacturer's directions. mRNA was prepared and reverse transcribed using the Express Direct system (Pierce, Rockford, Ill.). cDNA was amplified using primers specific for the disrupted Aβ fragment in mice on the black background (B6, B10, C2D, and B10 × C2D). The sense primer was 5′-TTCAAGGGCGAGTGCTACTT-3′, and the antisense primer was 5′-ACAGTGAATGGGGCTCTTCAG-3′. The primers amplify a 525-bp fragment in B6 and B10 mice, but not in C2D or B10 × C2D mice. A nondisrupted mRNA fragment was amplified for all mouse samples as a control using the sense primer 5′-CCACCACAACACTCTGGTCTGTTC-3′ and the antisense primer 5′-TGCCGCTCAACATCTTGCTCCG-3′. These primers amplify a 278-bp fragment from all mouse strains. PCR conditions were as follows: denaturing reverse transcriptase at 95°C for 5 min; 35 cycles of 95°C for 1 min, 58°C for 1 min, and 72°C for 2 min; and a final extension at 72°C for 5 min. Amplified DNA was separated on 1.5 to 2% agarose gels and visualized with ethidium bromide.
(ii) TLR4.
B6, B10, and B10 × C2D mice were assessed for expression of TLR4 mRNA because black mice have a gene deletion that does not allow the mRNA expression (26, 28). For this assessment RT-PCR was performed and a mouse with the presence or absence of a 476-bp cDNA fragment was interpreted as a TLR4Lps-n mouse or TLR4Lps-del mouse, respectively. For this assessment the sense primers 5′-GTATATGTGAACATCAGAAATTCCT-3′ and the antisense primer 5′-CATGTTTGAGCAATCTCATATTCAA-3′ were utilized. The RT-PCR conditions were previously described (34).
C2D macrophage cell line.
The C2D macrophage cell line was created by our group (3).
These cells were derived from C2D mouse bone marrow and selected in the presence of colony stimulating factor 1. After several crisis periods, the cells lost their dependence on colony stimulating factor 1, and the differentiated cells were characterized to have macrophage properties (3). These cells have the MHCII−/− and TLR4Lps-n genotype.
Adoptive transfer. (i) C2D whole bone marrow.
Long bones were recovered from 6-week-old female C2D mice, and the marrow cavity was flushed with sterile heparin-phosphate-buffered saline (h-PBS; 100 U/ml) solution. Red blood cells were lysed in the cell suspension with 0.17 M NH4Cl. Cells were centrifuged and washed two times to remove serum components and resuspended in warm (37°C) sterile h-PBS solution. Mice received intraperitoneal (i.p.) injections of 2.0 × 107 cells per 500 μl of sterile h-PBS solution or intravenous injections through the lateral tail vein of 2.0 × 107 cells per mouse in 200 μl of h-PBS solution. Mice were rested for 7 days before experimentation.
(ii) C2D macrophage cell line.
C2D macrophages were grown in 150-mm-diameter tissue culture plates in Dulbecco's modified Eagle's medium supplemented with 10% OptiMEM (vol/vol), 0.3% l-glutamine (wt/vol), and 10% serum (vol/vol). Cells were dispersed from confluent cultures with trypsin and washed three times from plates with sterile h-PBS. Mice received intravenous or i.p. injections as previously described and were rested for 7 days before experimentation.
Bacteria.
P. pneumotropica was grown as previously described (9). Mice were intranasally infected with bacteria in log-phase growth by inducing gasping with exposure to a 50:50 carbon dioxide-oxygen mixture followed by the application of 25 μl of bacterial suspension (4.0 × 108 to 7.0 × 109 CFU per animal). Mice were monitored daily for the development of symptoms and were euthanized to assess lung infections at predetermined times.
Lung assessment and detection of P. pneumotropica.
Mice were euthanized and the lungs were removed. The percentage of pneumonic tissue for each lung lobe was determined with the use of a dissection microscope and multiplied by 2 (left lobe), 1 (right superior and right inferior lobes), or 0.5 (right middle and postcaval lobe) to account for the relative contribution of each lobe to the total pulmonary mass. The total lesion score is the sum of the individual lung scores of all the mice in each strain tested (9). Bacterial infection was determined by assay for P. pneumotropica DNA isolated from lung tissue by PCR using the P. pneumotropica-specific primers 5′-CGGAATAACTGGGCGTAA-3′ (upstream) and 5′-GTCTCCTTTGAGTTCCCGACC-3′ (downstream) as previously described (9). Densitometric comparisons between P. pneumotropica DNA and the mammalian housekeeping gene S14 DNA were performed on ethidium bromide-stained agarose gels. Detection of P. pneumotropica in this manner correlates with lung bacterial isolate CFU determinations (r2 > 0.75) (9).
Detection of inflammatory cells in BAL fluid.
Cells were collected by bronchoalveolar lavage (BAL) from six mice per treatment group of infected B10 and B10 × C2D mice 24, 48, 72, 120, and 240 h postinfection. Mice were infected as described above, and lavage was performed by cannulation of each trachea with a blunt-end needle tied in place with surgical thread. Lungs were infused with 1 ml of EDTA-PBS (0.2 g/liter; 25°C) using a 1-ml syringe and gently massaged as previously described (9). Cytospins were prepared using 2.0 × 105 cells from the BAL. Cells were stained using Quick-Dif (Biochemical Sciences, Swedesboro, N.J.), and differential counts were determined by scoring a minimum of 200 cells. Uninfected B10 and B10 × C2D mice were used to establish baseline cell population percentages.
Peroxidase assays were performed using 2 × 105 cells per well in 96-well flat-bottom plates (Costar, Corning, N.Y.). The presence of peroxidase was determined by addition of TM Blue (50 μl/well; INTERGEN, Milford, Mass.). The color reaction was allowed to develop approximately 10 min, 1 N HCl (50 μl/well) was added to stop the reaction, and plates were read using a Spectra Count apparatus (Packard Instrument Companies, Meriden, Conn.).
Detection of chemokines and cytokines.
Total RNA was extracted from cells using TRIzol according to the manufacturer's directions (Invitrogen). cDNA was amplified using primers specific for each chemokine. These were sense (5′-GGGATGAGAAGTTCCCAAATG-3′) and antisense (5′-CTCCAGCTGGAAGACTCCTCCCAG-3′) primers for tumor necrosis factor alpha (TNF-α) (436-bp product), sense (5′-CTCACCTGCTGCTACTCATTC-3′) and antisense (5′-GCTTGAGGTGGTTGTGGAAAA-3′) primers for JE (318-bp product), sense (5′-GCATTCAGTTCCAGGTCAGTG-3′) and antisense (5′-CCCAGCCAGGTGCATTTTCC-3′) primers for macrophage inflammatory protein 1α (MIP-1α) (102-bp product), sense (5′-AGTTTGCCTTGACCCTGAAGCC-3′) and antisense (5′-TGGGTGGGATGTAGCTAGTTCC-3′) primers for MIP-2 (465-bp product), and sense (5′-AACGGAGAAAGAAGACAGACTGCT-3′) and antisense (5′-GACGAGACCAGGAGAAACAGGG-3′) primers for KC (529-bp product). PCR conditions remained as previously described for MHCII screening. Densitometric comparisons between chemokine DNA and the β-actin DNA were performed on ethidium bromide-stained 2% agarose gels.
Statistics.
Differences between treatment groups were determined using the indicated test analyses with the StatMost statistical package (Dataxiom, Los Angeles, Calif.).
RESULTS
Reconstitution with TLR4-positive cells increases resistance to P. pneumotropica.
TLR4 has been well characterized as the primary recognition molecule of LPS of gram-negative bacteria (4, 7, 27). We have previously shown that B10 mice develop transient pneumonia after bacterial challenge with P. pneumotropica, whereas C2D mice (MHCII−/− TLR4Lps-n) cleared the infection with minimal symptoms (9). Since B10 mice are susceptible to infection with P. pneumotropica and are TLR4Lps-del, we tested the hypothesis that reconstitution of these mice with whole bone marrow from C2D mice would restore host immunity. B10 mice received an i.p. injection of whole bone marrow, while control mice received no transfer. Lungs were examined for evidence of infection by assaying for P. pneumotropica DNA at the peak of infection (day 5 after challenge). Mice that did not receive C2D whole bone marrow developed pneumonia. Recipients of C2D bone marrow had lower lesion scores and bacterial load (P < 0.02; Table 2) than control mice, indicating less severe pneumonia and greater protection after challenge. Since TLR4 is expressed on neutrophils, dendritic cells, monocytes, and macrophages (22), the cell type critical for protection from P. pneumotropica was unclear. Alveolar macrophages are the first host immune cells to come into contact with inhaled antigen (13, 14). We hypothesized that reconstitution of B10 mice with a TLR4-positive macrophage cell line, C2D macrophages (3), would reduce the infection. B10 mice received C2D macrophages, while control mice received no transfer prior to infection. Mice that did not receive C2D macrophages developed severe, transient pneumonia, while mice receiving transferred C2D macrophages ameliorated the infection completely as determined by lung lesion score or the presence of bacteria (P < 0.02; Table 3). These data indicate that TLR4-positive macrophages are important to host resistance in the early stages of pulmonary bacterial infection.
TABLE 2.
Assessment of P. pneumotropica-induced lung damage following adoptive transfer of C2D whole bone marrow to B10 mice
| Treatment | Assay | Lung assessment at peak infectiona after rest period (days)
|
|
|---|---|---|---|
| 7 | 60 | ||
| No transfer | Lesion score | 2.3b | 2.5b |
| Bacterial loadc | 0.5b | 0.6b | |
| Bone marrow transfer | Lesion score | 0.9 | 0 |
| Bacterial load | 0.2 | 0 | |
Lesion scores were determined at the peak infection time after intranasal infection by assessing lungs for evidence of bacterium-induced pneumonia as described in Materials and Methods. Mice were infected following a 7- or 60-day rest period.
Statistically different from value for bone marrow-recipient mice (Mann-Whitney test, P < 0.02; n = 18 mice per treatment group [three experiments combined]).
Bacterial load was determined semiquantitatively by using a densitometric ratio of P. pneumotropica DNA/S14 DNA and imaged with ethidium bromide after agarose gel electrophoresis as described in Materials and Methods.
TABLE 3.
Assessment of P. pneumotropica-induced lung damage following adoptive transfer of C2D macrophages to B10 mice
| Treatment | Assay | Lung assessment at peak infectiona after rest period (days)
|
|
|---|---|---|---|
| 7 | 60 | ||
| No transfer | Lesion score | 2.5b | 2.6b |
| Bacterial loadc | 0.4b | 0.5b | |
| C2D macrophage | |||
| transfer | Lesion score | 0 | 0 |
| Bacterial load | 0 | 0 | |
Lesion scores were determined at the peak infection time after intranasal infection by assessing lungs for evidence of bacterium-induced pneumonia as described in Materials and Methods. Mice were infected following a 7- or 60-day rest period.
Statistically different from value for C2D macrophage-recipient mice (Mann-Whitney test, P < 0.02; n = 12 mice per treatment group [two experiments combined]).
Bacterial load was determined semiquantitatively by using a densitometric ratio of P. pneumotropica DNA/S14 DNA and imaged with ethidium bromide after agarose gel electrophoresis as described in Materials and Methods.
B10 mice have CD4+ T cells and can mount an adaptive immune response to infection. To determine if C2D bone marrow and C2D macrophages are protective in the absence of CD4+ T cells, we adoptively transferred C2D bone marrow or C2D macrophages to B10 × C2D mice. B10 × C2D mice carry the MHCII −/− and TLR4Lps-del genotype; do not have CD4+ T cells, and develop severe, lethal pneumonia after experimental challenge (9). Control mice received no transfer or transfer with B10 or B10 × C2D bone marrow. Adoptive transfer with C2D bone marrow increased survival to 70% compared with ≤23% survival in control groups (P < 0.01; Table 4). In contrast, mice receiving C2D macrophages had an 85% survival rate (Table 4). The higher survival rate of B10 × C2D mice to whom we had adoptively transferred C2D macrophages was consistent with our findings in B10 mice and indicates that both whole bone marrow and C2D macrophages can protect mice in the absence of CD4+ T cells.
TABLE 4.
Survival of B10 × C2D mice after adoptive transfer and P. pneumotropica challenge
| Treatmentb | No. of survivors/total no. of mice challenged at days postchallengea
|
Final % survivalc | |||
|---|---|---|---|---|---|
| 5 | 8 | 13 | 21 | ||
| No transfer | 20/30 | 14/30 | 11/30 | 7/30 | 23 A |
| C2D Marrow | 9/10 | 7/10 | 7/10 | 7/10 | 70 B |
| B10 marrow | 6/10 | 6/10 | 4/10 | 2/10 | 20 A |
| B10 × C2D marrow | 7/10 | 4/10 | 2/10 | 1/10 | 10 A |
| C2D macrophage | 20/20 | 19/20 | 19/20 | 17/20 | 85 B |
Mice challenged intranasally with of 6.0 × 109 CFU of P. pneumotropica.
Mice subjected to adoptive transfer received 2.0 × 107 cells per mouse 7 days prior to P. pneumotropica challenge (four experiments combined).
Treatment groups with different letters are statistically different (Fisher's exact test, P < 0.01).
Long-term protection of adoptively transferred TLR4-positive cells.
TLR4 can be detected in B10 and B10 × C2D mice 62 days after adoptive transfer (Hart et al., submitted). We tested the hypothesis that bone marrow and C2D macrophages would enhance host resistance to P. pneumotropica following a 60-day prechallenge incubation period. To test this hypothesis, B10 and B10 × C2D mice were infected 60 days after adoptive transfer. B10 mice were assessed at the 5-day peak infection time (Tables 2 and 3), and B10 × C2D mice were assessed for survival (Table 5). Both whole bone marrow and C2D macrophages were effective in decreasing the severity of pneumonia (P < 0.02) in both B10 mice (Table 3) and B10 × C2D mice (Tables 4 and 5). B10 × C2D mice that received C2D macrophages demonstrated a 90% survival rate compared to the 30% survival rate in the group that did not receive adoptively transferred cells (P < 0.05; Table 5). These data indicate that both adoptively transferred cell types provide long-term protection from P. pneumotropica infection.
TABLE 5.
Survival of B10 × C2D mice 60 days post-C2D macrophage adoptive transfer with P. pneumotropica challenge
| Treatmentb | No. of survivors/total no. of mice challenged at days postchallengea
|
Final % survival | |||
|---|---|---|---|---|---|
| 5 | 8 | 13 | 21 | ||
| No transfer | 15/20 | 11/20 | 8/20 | 6/20 | 30c |
| Transfer | 20/20 | 20/20 | 18/20 | 18/20 | 90c |
Mice challenged intranasally with of 6.2 × 109 CFU of P. pneumotropica.
Mice subjected to adoptive transfer received 2.0 × 107 cells per mouse and were allowed to rest 60 days following adoptive transfer of C2D macrophages.
Treatment groups statistically different (Fisher's exact test, P < 0.05).
Impact of adoptively transferred TLR4-positive cells on pulmonary inflammation.
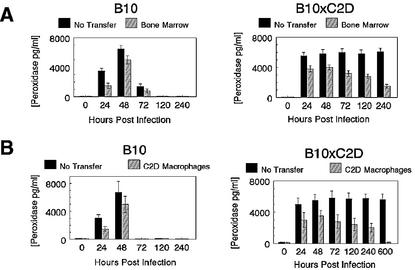
Because whole bone marrow and C2D macrophages protected both B10 and B10 × C2D mice from P. pneumotropica-induced pneumonia, we determined the impact of transferred cells on lung inflammation before and after infection. Macrophages were the predominant cell type in uninfected B10 and B10 × C2D mice with or without adoptive transfer (Tables 6 and 7), indicating that bone marrow and C2D macrophages did not cause inflammation or upset the normal alveolar macrophage balance. Cells from BAL fluid were collected at 24, 48, 72, 120, and 240 h post-intranasal bacterial challenge. B10 mice that received adoptive transfer of C2D macrophages had less dramatic neutrophil inflammation in the alveoli compared to controls at 24 h (P < 0.05), with peak neutrophilia occurring between 48 and 72 h for both bone marrow and C2D macrophage treatment groups (Table 6). The increased influx of neutrophils at 24 h correlated with increased peroxidase activity in lung lavage cells for all mice at that time (Fig. 1). Therefore, adoptive transfer of C2D macrophages did affect early lung inflammation. B10 × C2D mice that received adoptive transfer also maintained a normal distribution of macrophages in the lung, similar to that observed in controls (Table 7). After infection, mice that received no transfer had almost twice the percentage of neutrophils compared to transfer groups. The neutrophilia persisted for 25 days in control mice, whereas neutrophilia in adoptive transfer mice began to decline toward normal levels (Table 7). The higher percentage of neutrophils in non-adoptive-transfer mice correlated with peroxidase activity of BAL from B10 × C2D mice (Fig. 1). No differences in the number of eosinophils and lymphocytes present in the BAL were noticed in either test group (data not shown). These data indicate that non-adoptive-transfer B10 × C2D mice have chronic, unresolved pneumonia, while the adoptive-transfer recipients have less dramatic neutrophilia, consistent with a significantly higher survival rate of whole bone marrow and C2D macrophage recipients.
TABLE 6.
Inflammatory cells present in BAL fluid of B10 mice with C2D whole bone marrow or C2D macrophage adoptive transfer and P. pneumotropica challenge
| Time (h) postinfectiona | No. of inflammatory cells present (mean ± SEM) afterb:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Whole bone marrow transfer
|
C2D macrophage transfer
|
|||||||
| Macrophage
|
Neutrophil
|
Macrophage
|
Neutrophil
|
|||||
| No transfer | Transferc | No transfer | Transfer | No transfer | Transfer | No transfer | Transfer | |
| 0 | 94 ± 2 | 97 ± 1 | 2 ± 1 | 1 ± 0 | 98 ± 3 | 98 ± 2 | 2 ± 1 | 1 ± 0 |
| 24 | 56 ± 2 | 64 ± 2 | 32 ± 1 | 29 ± 1 | 58 ± 2 | 72 ± 2 | 30 ± 2 | 19 ± 1 |
| 48 | 50 ± 2 | 54 ± 1 | 36 ± 2 | 34 ± 2 | 50 ± 4 | 54 ± 2 | 36 ± 3 | 34 ± 2 |
| 72 | 44 ± 3 | 53 ± 2 | 49 ± 3 | 41 ± 1 | 41 ± 4 | 50 ± 2 | 49 ± 4 | 37 ± 1 |
| 120 | 56 ± 2 | 59 ± 2 | 33 ± 2 | 31 ± 2 | 57 ± 2 | 61 ± 2 | 32 ± 2 | 28 ± 3 |
| 240 | 67 ± 3 | 68 ± 2 | 26 ± 3 | 22 ± 2 | 78 ± 4 | 84 ± 1 | 17 ± 4 | 10 ± 2 |
BAL fluid was collected from six animals per treatment group at 24, 48, 72, 120, 240 h post-bacterial challenge.
Values based on six mice per treatment group. Percentage of cells determined by scoring ≥200 cells per mouse sample. Significant differences (P < 0.05) between transfer and no transfer at 0, 24, and 72 has determined by χ2.
Recipient animals received injections of 2.0 × 107 cells per mouse i.p.
TABLE 7.
Inflammatory cells present in BAL fluid of B10 × C2D mice with C2D whole bone marrow or C2D macrophage adoptive transfer and P. pneumotropica challenge
| Time (h) postinfectiona | No. of inflammatory cells (mean ± SEM) present afterb:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Whole bone marrow transfer
|
C2D macrophage transfer
|
|||||||
| Macrophage
|
Neutrophil
|
Macrophage
|
Neutrophil
|
|||||
| No transfer | Transferc | No transfer | Transfer | No transfer | Transfer | No transfer | Transfer | |
| 0 | 97 ± 1 | 98 ± 2 | 1 ± 0 | 1 ± 0 | 96 ± 2 | 98 ± 2 | 2 ± 1 | 2 ± 0 |
| 24 | 35 ± 2 | 62 ± 2 | 57 ± 2 | 33 ± 1 | 40 ± 2 | 60 ± 2 | 54 ± 2 | 35 ± 1 |
| 48 | 35 ± 4 | 62 ± 2 | 57 ± 3 | 34 ± 2 | 35 ± 4 | 60 ± 2 | 56 ± 3 | 36 ± 2 |
| 72 | 35 ± 3 | 65 ± 2 | 57 ± 7 | 28 ± 1 | 34 ± 5 | 65 ± 2 | 58 ± 7 | 30 ± 1 |
| 120 | 40 ± 2 | 65 ± 2 | 51 ± 2 | 30 ± 3 | 40 ± 2 | 65 ± 2 | 53 ± 2 | 30 ± 3 |
| 240 | 42 ± 4 | 72 ± 1 | 55 ± 4 | 28 ± 2 | 41 ± 4 | 70 ± 1 | 56 ± 4 | 30 ± 2 |
BAL fluid was collected from six animals per treatment group at 24, 48, 72, 120, 240 h post-bacterial challenge.
Values based on six mice per treatment group. Percentage of cells determined by scoring ≥200 cells per mouse sample. Significant differences (P < 0.05) between transfer and no transfer at 0, 24, and 72 h as determined by χ2.
Recipient animals received injections of 2.0 × 107 cells per mouse i.p.
FIG. 1.
Peroxidase concentration in BAL fluids of B10 and B10 × C2D mice after adoptive transfer with whole bone marrow or C2D macrophages and intranasal P. pneumotropica challenge after a 7-day rest period. Lungs were washed with PBS-EDTA, and peroxidase levels were measured using substrate conversion with TM Blue. (A) Peroxidase concentration in BAL fluids of B10 mice; (B) peroxidase concentration in BAL fluids of B10 × C2D mice. Error bars, standard deviations.
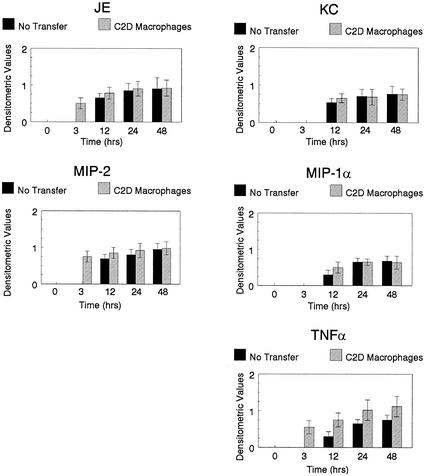
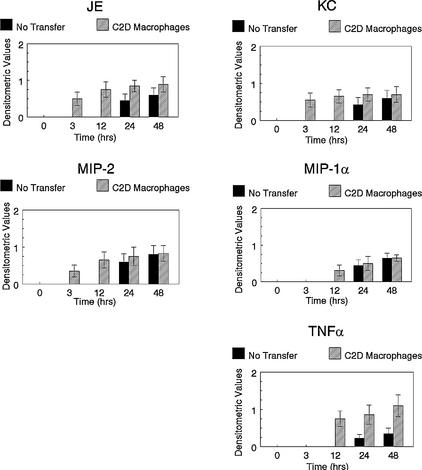
Cytokines are secreted by macrophages to help regulate inflammation and bacterial clearance. We have demonstrated previously that C2D macrophages transcribe cytokines like TNF-α in response to the in vivo environment (Hart et al., submitted). We hypothesized that the production of cytokines by C2D macrophages may have a direct impact on the transcription of chemokines and cytokines of host macrophages. Mice to whom we had adoptively transferred C2D macrophages were challenged with P. pneumotropica, the lungs were harvested at various time points, and cytokine transcription was determined by RT-PCR. A densitometric ratio was determined as described in Materials and Methods. JE and MIP-2 were transcribed earlier in adoptive-transfer B10 and B10 × C2D mice compared to control mice (Fig. 2 and 3). Similarly, TNF-α transcription levels increased sooner in mice that received adoptive transfer compared to non-adoptive-transfer mice. KC and MIP-1α were also transcribed earlier in B10 × C2D mice that received C2D macrophage transfer compared to non-adoptive-transfer controls (Fig. 3). However, similar differences were not apparent in B10 mice. Therefore, adoptively transferred C2D macrophages may impact host inflammatory responsiveness by altering the production of chemokines and cytokines in the lung.
FIG. 2.
Cytokine transcription in the lungs of B10 mice. Mice were euthanized at various times following bacterial challenge. Lungs were removed, and RNA was collected. Cytokine transcription levels were determined semiquantitatively using cytokine-specific primers, and a densitometric ratio (cytokine product/β-actin product) was determined. Error bars, standard deviations.
FIG. 3.
Cytokine transcription in the lungs of B10 × C2D mice. Mice were euthanized at various times following bacterial challenge. Lungs were removed, and RNA was collected. Cytokine transcription levels were determined semiquantitatively using cytokine-specific primers, and a densitometric ratio (cytokine product/β-actin product) was determined. Error bars, standard deviations.
DISCUSSION
Reconstitution of TLR4Lps-del mice with TLR4-positive bone marrow cells or a TLR4-positive macrophage cell line restored host resistance to lung infections caused by P. pneumotropica. C2D macrophages provided protection earlier after adoptive transfer compared to whole bone marrow cells. This was probably due to the number and types of cells adoptively transferred. Bone marrow is composed of undifferentiated stem cells as well as mature cell types of various lineages, including cells that do not express TLR4. This would limit the number of cells that could recognize gram-negative bacteria and participate in the protective response. Furthermore, the undifferentiated cells would affect the timely recognition of bacteria. In contrast, C2D macrophages are already differentiated, express TLR4, and are capable of an immediate response.
Adoptively transferred C2D macrophages do not cause inflammation or upset normal alveolar macrophage distribution. The absence of detrimental pathology allows long-term survival of C2D cells in vivo (greater than 2 months) with a continued contribution to host resistance (Hart et al., submitted). Our study conclusively demonstrates that macrophages adapted to long-term in vitro culture can be reintroduced in vivo and contribute significantly to host resistance.
The adoptive transfer of C2D macrophages affects pulmonary inflammation. Mice given C2D macrophages had fewer neutrophils in the alveoli than control mice at 24 h postinfection (P < 0.05). Although the presence of polymorphonuclear leukocytes (PMNs) at 24 h correlated with increased peroxidase activity of lung lavage cells, there was decreased peroxidase at later times in B10 mice in spite of the presence of PMNs in the BAL. This suggests that immigration of fresh PMNs diminished and that older degranulated PMNs remained in the alveoli of B10 mice. There was a better correlation between PMNs and peroxidase activation in B10 × C2D mice, where it took several weeks for PMN removal. The return to normal baseline cell concentrations occurred faster in protected mice to whom cells had been adoptively transferred. Therefore, C2D macrophages probably limited the infection and ameliorated the intensity of subsequent inflammation. Moreover, the presence of CD4+ T cells seems to limit inflammation by PMN. It would appear that in the normal situation, both T cells and macrophages work to limit infection.
C2D macrophages may enhance host resistance because they produce some cytokines (3). C2D macrophages constitutively transcribe the chemokines JE and KC, and transcription is enhanced with LPS stimulation (Hart et al., submitted). This is consistent with the heightened expression of these early chemokines in thioglycolate-elicited peritoneal macrophages (36). Administration of human monocyte-chemotactic and activating factor, a homologue of murine JE, enhances mouse survival after experimental challenge with Pseudomonas aeruginosa or Salmonella enterica serovar Typhimurium (23). In vitro and in vivo experiments demonstrated that administration of monocyte-chemotactic and activating factor enhanced phagocytosis and bactericidal activity in a dose-dependent manner (23). Therefore, early expression of JE by adoptively transferred C2D macrophages may stimulate recipient immunity. This hypothesis is supported by our observation that C2D macrophages accelerated the kinetics of in vivo lung cytokine production after infection. Perhaps this early burst of activity is the key to limiting infection. In addition, we previously showed that C2D macrophages transcribe TNF-α after exposure to the in vivo microenvironment (Hart et al., submitted), even though in vitro-cultured cells make little TNF-α (3). Production of TNF-α by adoptively transferred C2D macrophages could also directly stimulate recipient macrophages and influence subsequent resistance. It may be a combination of cytokine production by adoptively transferred macrophages and recipient responses that accounts for the effective resistance in susceptible mouse strains. Additional studies will be necessary to isolate the key components of resistance after adoptive transfer.
Many groups have adoptively transferred macrophages for the treatment of disease (1, 10, 12, 18, 25). Freudenberg et al. demonstrated that bone marrow-derived macrophages could restore a lethal LPS response in C3H/HeJ mice (12) and proved that reconstitution of macrophage function was possible using adoptive transfer. Nevertheless, there are few data on long-term host reconstitution with established macrophage cell lines. In one study J774A.1 macrophages, transfected with murine gamma interferon and enhanced fluorescent green protein, partially restored pulmonary immune function of SCID mice (35). However, the transfected macrophages had decreased expression of murine gamma interferon and enhanced fluorescent green protein over time, suggesting that J774.1 transfer did not offer long-term protection. Similarly, Nishihara et al. found that their macrophage cell line only functioned for 20 days in vivo (24).
A common complication of adoptive transfer of macrophage cell lines is neoplasia. Macrophage cell lines continuously grown in culture are not growth factor regulated and can be tumorigenic (2, 29). Introduction of a simian virus 40-transformed macrophage cell line into both B6 and Aβ knockout mice caused progressively growing tumors in both mouse strains (3). Therefore, the nontumorigenic cell line, C2D macrophages, offer a unique tool for long-term in vivo studies.
The ability of C2D macrophages to restore host resistance to P. pneumotropica reaffirms the important role of macrophages in pulmonary health. Since both whole bone marrow and C2D macrophages are MHCII−/− and can protect B10 × C2D mice in the absence of CD4+ T cells, activation through MHCII or by helper T cells is not as important as stimulation through the TLR4 pathway. The more-dramatic effects of C2D macrophages on B10 × C2D mice, compared to B10 mice, probably reflect the reversal of a more-dramatic immunodeficiency and effectively demonstrates that macrophages alone restore host resistance to P. pneumotropica. However, the more-efficient clearance of P. pneumotropica in B10 mice compared to B10 × C2D mice with or without adoptive transfer shows the contribution of helper T cells in normal host resistance. Reintroduction of TLR4-positive macrophages may be one method for prevention and treatment of persistent pulmonary gram-negative bacterial infections.
Although macrophage therapy may prove beneficial for some bacterial infections, it may not be universally effective. For example, Lettinga et al. found that TLR4 was not important for pulmonary resistance against the gram-negative bacterium Legionella pneumophila (20). Leemans et al. found that the presence of normal macrophages not only facilitated capture of Mycobacterium tuberculosis but also the growth of the bacilli (19). Depletion of alveolar macrophages resulted in a modicum of pulmonary protection (19). Therefore, the complex role of alveolar macrophages and TLR4 may be dependent on the type of bacteria present in the pulmonary environment. P. pneumotropica is a pyogenic, extracellular bacterium. L. pneumophila and M. tuberculosis grow intracellularly. The ability of C2D macrophages to effectively restore host immunity appears to be dependent on the presence of TLR4, cellular activation through this pathway, and the subsequent production of cytokines. Further research should explore the full protective potential of C2D macrophages against both intracellular and extracellular microorganisms.
Acknowledgments
We thank Donna Rogers for her laboratory assistance with these studies. We also thank Sherry Fleming (Walter Reed Army Institute of Research, Washington, D.C.) for chemokine and cytokine primer sequences.
This work was supported by The Kansas NASA Space Grant Consortium; NASA grants NAGW-1197, NAG2-1274, and NCC5-168; U.S. Department of Agriculture Animal Health Funds section 1433, grant 4-81895; and NIH grants RR16475 and AI050785.
Editor: J. D. Clements
Footnotes
This is publication number 03-12-J of the Kansas Agricultural Experiment Station.
REFERENCES
- 1.Abe, T., K. Yoshimura, and Y. Nawa. 1992. Restoration of the defective natural defence of beige mice against tissue-migrating larvae of Strongyloides ratti by transfer with normal peritoneal cells. Int. J. Parasitol. 22:545-547. [DOI] [PubMed] [Google Scholar]
- 2.Altenschmidt, U., P. Ricciardi-Castagnoli, M. Modolell, H. Otto, K. H. Wiesmuller, G. Jung, and M. M. Simon. 1996. Bone marrow-derived macrophage lines and immortalized cloned macrophage and dendritic cells support priming of Borrelia burgdorferi-specific T cell responses in vitro and/or in vivo. Immunol. Lett. 50:41-49. [DOI] [PubMed] [Google Scholar]
- 3.Beharka, A. A., J. W. Armstrong, and S. K. Chapes. 1998. Macrophage cell lines derived from major histocompatibility complex II-negative mice. In Vitro Cell. Dev. Biol. Anim. 34:499-507. [DOI] [PubMed] [Google Scholar]
- 4.Beutler, B., X. Du, and A. Poltorak. 2001. Identification of Toll-like receptor 4 (Tlr4) as the sole conduit for LPS signal transduction: genetic and evolutionary studies. J. Endotoxin Res. 7:277-280. [PubMed] [Google Scholar]
- 5.Beutler, B., and A. Poltorak. 2000. Positional cloning of Lps, and the general role of toll-like receptors in the innate immune response. Eur. Cytokine Netw. 11:143-152. [PubMed] [Google Scholar]
- 6.Beutler, B., and A. Poltorak. 2000. The search for Lps: 1993-1998. J. Endotoxin Res. 6:269-293. [PubMed] [Google Scholar]
- 7.Beutler, B., and A. Poltorak. 2001. The sole gateway to endotoxin response: how LPS was identified as Tlr4, and its role in innate immunity. Drug Metab. Dispos. 29:474-478. [PubMed] [Google Scholar]
- 8.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 9.Chapes, S. K., D. A. Mosier, A. D. Wright, and M. L. Hart. 2001. MHCII, Tlr4 and Nramp1 genes control host pulmonary resistance against the opportunistic bacterium Pasteurella pneumotropica. J. Leukoc. Biol. 69:381-386. [PubMed] [Google Scholar]
- 10.Ding, L., S. Lu, R. Batchu, R. L. Saylors III, and N. Munshi. 1999. Bone marrow stromal cells as a vehicle for gene transfer. Gene Ther. 6:1611-1616. [DOI] [PubMed] [Google Scholar]
- 11.Fels, A. O., and Z. A. Cohn. 1986. The alveolar macrophage. J. Appl. Physiol. 60:353-369. [DOI] [PubMed] [Google Scholar]
- 12.Freudenberg, M. A., D. Keppler, and C. Galanos. 1986. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect. Immun. 51:891-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green, G. M. 1968. Pulmonary clearance of infectious agents. Annu. Rev. Med. 19:315-336. [DOI] [PubMed] [Google Scholar]
- 14.Greene, R. E. 1968. Unresolving pneumonia. JAMA 203:287-289. [PubMed] [Google Scholar]
- 15.Grusby, M. J., and L. H. Glimcher. 1995. Immune responses in MHC class II-deficient mice. Annu. Rev. Immunol. 13:417-435. [DOI] [PubMed] [Google Scholar]
- 16.Grusby, M. J., R. S. Johnson, V. E. Papaioannou, and L. H. Glimcher. 1991. Depletion of CD4+ T cells in major histocompatibility complex class II-deficient mice. Science 253:1417-1420. [DOI] [PubMed] [Google Scholar]
- 17.Jones, B. W., T. K. Means, K. A. Heldwein, M. A. Keen, P. J. Hill, J. T. Belisle, and M. J. Fenton. 2001. Different Toll-like receptor agonists induce distinct macrophage responses. J. Leukoc. Biol. 69:1036-1044. [PubMed] [Google Scholar]
- 18.Kitamura, M. 1999. Adoptive transfer of genetically modified macrophages elucidated TGF-beta-mediated ′self-defence' of the glomerulus against local action of macrophages. Nephrol. Dial. Transplant. 14:35-38. [DOI] [PubMed] [Google Scholar]
- 19.Leemans, J. C., N. P. Juffermans, S. Florquin, N. van Rooijen, M. J. Vervoordeldonk, A. Verbon, S. J. van Deventer, and T. van der Poll. 2001. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J. Immunol. 166:4604-4611. [DOI] [PubMed] [Google Scholar]
- 20.Lettinga, K. D., S. Florquin, P. Speelman, R. van Ketel, T. van der Poll, and A. Verbon. 2002. Toll-like receptor 4 is not involved in host defense against pulmonary Legionella pneumophila infection in a mouse model. J. Infect. Dis. 186:570-573. [DOI] [PubMed] [Google Scholar]
- 21.Murray, J. 1986. The normal lung, 2nd ed., p. 95-128. Centaurs, Philadelphia, Pa.
- 22.Muzio, M., N. Polentarutti, D. Bosisio, M. K. Prahladan, and A. Mantovani. 2000. Toll-like receptors: a growing family of immune receptors that are differentially expressed and regulated by different leukocytes. J. Leukoc. Biol. 67:450-456. [DOI] [PubMed] [Google Scholar]
- 23.Nakano, Y., T. Kasahara, N. Mukaida, Y. C. Ko, M. Nakano, and K. Matsushima. 1994. Protection against lethal bacterial infection in mice by monocyte-chemotactic and -activating factor. Infect. Immun. 62:377-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishihara, K., R. F. Barth, N. Wilkie, J. C. Lang, Y. Oda, H. Kikuchi, M. P. Everson, and M. T. Lotze. 1995. Increased in vitro and in vivo tumoricidal activity of a macrophage cell line genetically engineered to express IFN-gamma, IL-4, IL-6, or TNF-alpha. Cancer Gene Ther. 2:113-124. [PubMed] [Google Scholar]
- 25.Pillai, C. R., and C. U. Devi. 2000. Role of macrophages in experimental malaria. VII. Studies on adoptive transfer of macrophages. J. Commun. Dis. 32:129-135. [PubMed] [Google Scholar]
- 26.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 27.Poltorak, A., I. Smirnova, X. He, M. Y. Liu, C. Van Huffel, O. McNally, D. Birdwell, E. Alejos, M. Silva, X. Du, P. Thompson, E. K. Chan, J. Ledesma, B. Roe, S. Clifton, S. N. Vogel, and B. Beutler. 1998. Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol. Dis. 24:340-355. [DOI] [PubMed] [Google Scholar]
- 28.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ralph, P. 1981. Continuous macrophage cell lines: their use in the study of induced and constitutive macrophage properties and cytotoxicity. Lymphokines 4:175-195. [Google Scholar]
- 30.Reynolds, H. 1989. Normal and defective respiratory host defenses, p. 1-33. In J. Pennington (ed.), Respiratory infections: diagnosis and management, 2nd ed. Raven Press, New York, N.Y.
- 31.Sibille, Y., and H. Y. Reynolds. 1990. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am. Rev. Respir. Dis. 141:471-501. [DOI] [PubMed] [Google Scholar]
- 32.Vallance, B. A., F. Galeazzi, S. M. Collins, and D. P. Snider. 1999. CD4 T cells and major histocompatibility complex class II expression influence worm expulsion and increased intestinal muscle contraction during Trichinella spiralis infection. Infect. Immun. 67:6090-6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Furth, R. 1988. Phagocytic cell: development and distribution of mononuclear phagocytes in normal steady state and inflammation, p. 281-295. In J. I. Gallin, I. M. Goldstein, and R. Snyderman (ed.), Inflammation: basic principles and clinical correlates. Raven Press, Ltd., New York, N.Y.
- 34.Wright, A. D., and S. K. Chapes. 1999. LPS sensitivity in recombinant mice lacking functional alleles at MHCII, LPS, and Nramp1 genes. J. Endotoxin Res. 5:297-305. [Google Scholar]
- 35.Wu, M., S. Hussain, Y. H. He, R. Pasula, P. A. Smith, and W. J. Martin, II. 2001. Genetically engineered macrophages expressing IFN-gamma restore alveolar immune function in scid mice. Proc. Natl. Acad. Sci. USA 98:14589-14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu, S. F., T. J. Koerner, and D. O. Adams. 1990. Gene regulation in macrophage activation: differential regulation of genes encoding for tumor necrosis factor, interleukin-1, JE, and KC by interferon-gamma and lipopolysaccharide. J. Leukoc. Biol. 48:412-419. [DOI] [PubMed] [Google Scholar]