Abstract
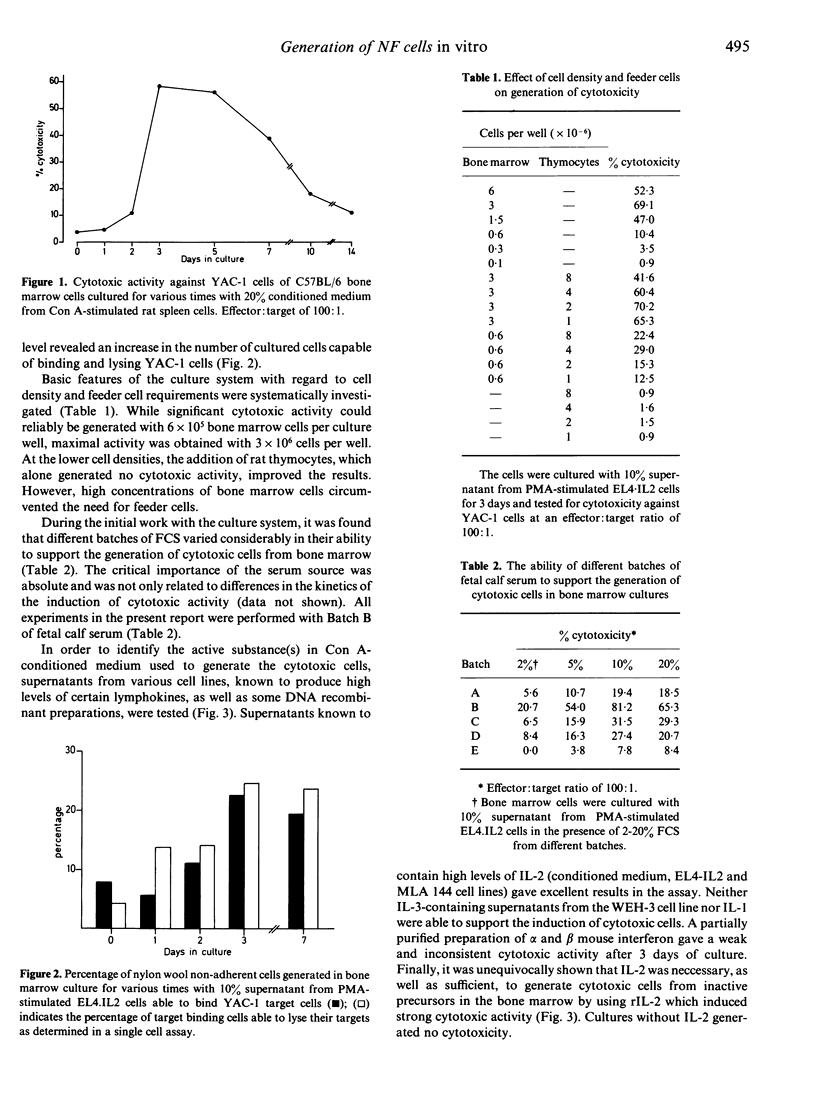
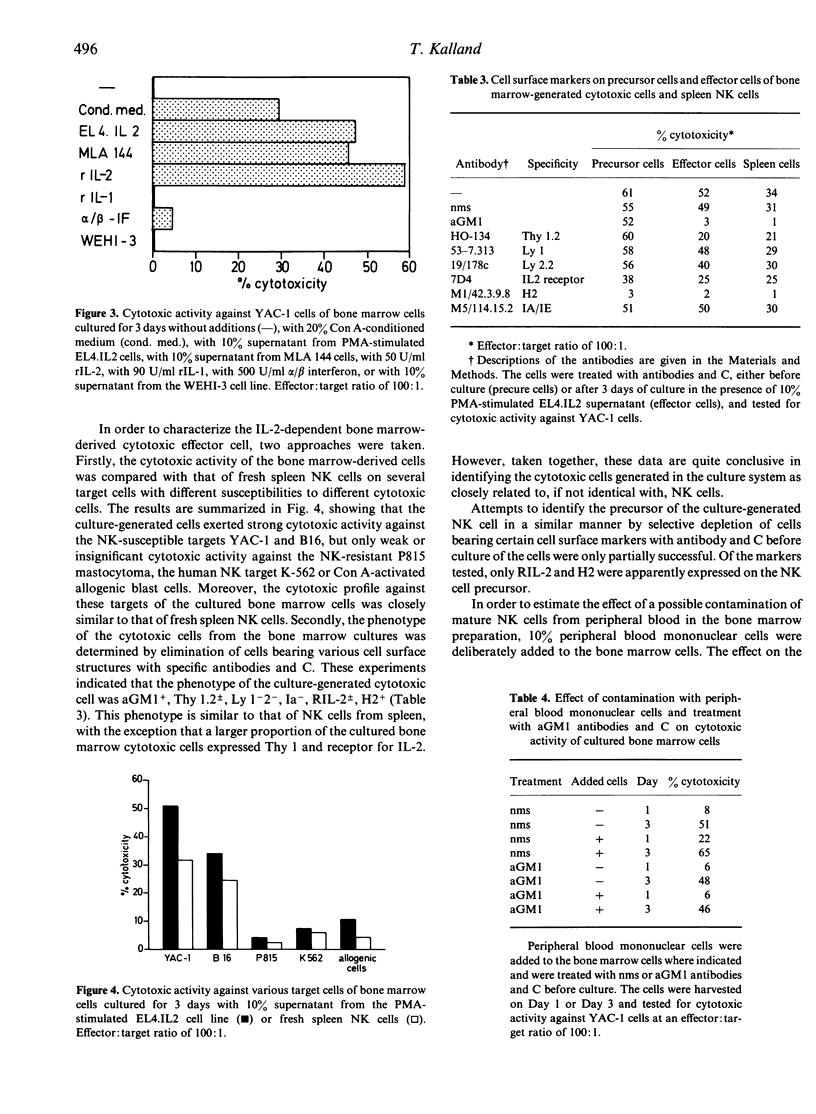
A simple and highly reproducible bone marrow culture system for the generation of cytolytically active NK cells from immature precursors in the bone marrow is described. The NK cells can be generated with various sources of IL-2, including Con A-conditioned medium, supernatants from IL-2-producing cell lines and recombinant IL-2. Neither IL-1, IL-3 nor alpha/beta interferon induced significant cytotoxicity in bone marrow cells. Identification of the cytotoxic cells as NK cells was based on their phenotypic characteristics (aGM1+, Thy 1 +/-, Ly 1-2- X Ia-, RIL-2 +/-, H2+), as well as their spectrum of target specificity. The deliberate addition of peripheral blood mononuclear cells as a source of mature NK cells and elimination of cells expressing markers specific for mature NK cells indicated that the generated NK cells were descendants of precursors of NK cells harboured in the bone marrow and not derived from mature NK cells contaminating the bone marrow preparations. Thus, it was shown that not only functionally active NK cells but also their precursors are highly dependent on IL-2 for differentiation and growth. This culture system should be helpful in studying the origin of NK cells in relation to other cell lineages as well as the regulation of the maturation of NK cells from their precursors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Brooks C. G. Reversible induction of natural killer cell activity in cloned murine cytotoxic T lymphocytes. Nature. 1983 Sep 8;305(5930):155–158. doi: 10.1038/305155a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski J. F., Woda B. A., Habu S., Okumura K., Welsh R. M. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983 Sep;131(3):1531–1538. [PubMed] [Google Scholar]
- Djeu J. Y., Lanza E., Pastore S., Hapel A. J. Selective growth of natural cytotoxic but not natural killer effector cells in interleukin-3. Nature. 1983 Dec 22;306(5945):788–791. doi: 10.1038/306788a0. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Fuller-Farrar J., Simon P. L., Hilfiker M. L., Stadler B. M., Farrar W. L. Thymoma production of T cell growth factor (Interleukin 2). J Immunol. 1980 Dec;125(6):2555–2558. [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E. A., Ramsey K. M., Mazumder A., Wilson D. J., Djeu J. Y., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. II. Precursor phenotype is serologically distinct from peripheral T lymphocytes, memory cytotoxic thymus-derived lymphocytes, and natural killer cells. J Exp Med. 1983 Mar 1;157(3):884–897. doi: 10.1084/jem.157.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E. A., Thoma J. A., Bonavida B. Mechanism of cell-mediated cytotoxicity at the single cell level. II. Evidence for first-order kinetics of T cell-mediated cytolysis and for heterogeneity of lytic rate. J Immunol. 1979 Dec;123(6):2870–2877. [PubMed] [Google Scholar]
- Haller O., Kiessling R., Orn A., Wigzell H. Generation of natural killer cells: an autonomous function of the bone marrow. J Exp Med. 1977 May 1;145(5):1411–1416. doi: 10.1084/jem.145.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M., Beran M., Andersson B., Kiessling R. Inhibition of in vitro granulopoiesis by autologous allogeneic human NK cells. J Immunol. 1982 Jul;129(1):126–132. [PubMed] [Google Scholar]
- Hansson M., Kiessling R., Andersson B. Human fetal thymus and bone marrow contain target cells for natural killer cells. Eur J Immunol. 1981 Jan;11(1):8–12. doi: 10.1002/eji.1830110103. [DOI] [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E. Destruction of Trypanosoma cruzi by Natural killer cells. Science. 1982 Oct 15;218(4569):295–296. doi: 10.1126/science.6812218. [DOI] [PubMed] [Google Scholar]
- Hämmerling G. J., Hämmerling U., Flaherty L. Qat-4 and Qat-5, new murine T-cell antigens governed by the Tla region and identified by monoclonal antibodies. J Exp Med. 1979 Jul 1;150(1):108–116. doi: 10.1084/jem.150.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalland T., Alm G., Stålhandshe T. Augmentation of mouse natural killer cell activity by LS 2616, a new immunomodulator. J Immunol. 1985 Jun;134(6):3956–3961. [PubMed] [Google Scholar]
- Kalland T. Exposure of neonatal female mice to diethylstilbestrol persistently impairs NK activity through reduction of effector cells at the bone marrow level. Immunopharmacology. 1984 Apr;7(2):127–134. doi: 10.1016/0162-3109(84)90062-6. [DOI] [PubMed] [Google Scholar]
- Kalland T., Forsberg J. G. 3-methylcholanthrene: transient inhibition of the lytic step of mouse natural killer cells. J Natl Cancer Inst. 1983 Aug;71(2):385–390. [PubMed] [Google Scholar]
- Kalland T., Forsberg J. G. Natural killer cell activity and tumor susceptibility in female mice treated neonatally with diethylstilbestrol. Cancer Res. 1981 Dec;41(12 Pt 1):5134–5140. [PubMed] [Google Scholar]
- Kalland T. Inhibition of T lymphocyte activation by estramustine: dose-dependent interference with IL-2 production and later proliferation events. Immunopharmacology. 1985 Apr;9(2):65–71. doi: 10.1016/0162-3109(85)90001-3. [DOI] [PubMed] [Google Scholar]
- Kalland T. Reduced natural killer activity in female mice after neonatal exposure to diethylstilbestrol. J Immunol. 1980 Mar;124(3):1297–1300. [PubMed] [Google Scholar]
- Kasai M., Yoneda T., Habu S., Maruyama Y., Okumura K., Tokunaga T. In vivo effect of anti-asialo GM1 antibody on natural killer activity. Nature. 1981 May 28;291(5813):334–335. doi: 10.1038/291334a0. [DOI] [PubMed] [Google Scholar]
- Klimpel G. R., Sarzotti M., Reyes V. E., Klimpel K. D. Characterization of cytotoxic cells generated from in vitro cultures of murine bone marrow cells. Cell Immunol. 1985 Apr 15;92(1):1–13. doi: 10.1016/0008-8749(85)90059-0. [DOI] [PubMed] [Google Scholar]
- Koo G. C., Peppard J. R., Mark W. H. Natural killer cells generated from bone marrow culture. J Immunol. 1984 May;132(5):2300–2304. [PubMed] [Google Scholar]
- Kumar V., Ben-Ezra J., Bennett M., Sonnenfeld G. Natural killer cells in mice treated with 89strontium: normal target-binding cell numbers but inability to kill even after interferon administration. J Immunol. 1979 Oct;123(4):1832–1838. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lohmann-Matthes M. L., Domzig W., Roder J. Promonocytes have the functional characteristics of natural killer cells. J Immunol. 1979 Oct;123(4):1883–1886. [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Seaman W. E., Blackman M. A., Gindhart T. D., Roubinian J. R., Loeb J. M., Talal N. beta-Estradiol reduces natural killer cells in mice. J Immunol. 1978 Dec;121(6):2193–2198. [PubMed] [Google Scholar]
- Stutman O., Paige C. J., Figarella E. F. Natural cytotoxic cells against solid tumors in mice. I. Strain and age distribution and target cell susceptibility. J Immunol. 1978 Nov;121(5):1819–1826. [PubMed] [Google Scholar]
- Suzuki R., Handa K., Itoh K., Kumagai K. Natural killer (NK) cells as a responder to interleukin 2 (IL 2). I. Proliferative response and establishment of cloned cells. J Immunol. 1983 Feb;130(2):981–987. [PubMed] [Google Scholar]
- Talmadge J. E., Meyers K. M., Prieur D. J., Starkey J. R. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980 Apr 17;284(5757):622–624. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Matsumoto-Kobayashi M., Clark S. C., Seehra J., London L., Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984 Oct 1;160(4):1147–1169. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung Y. P., Okumura K., Moore M. A. Generation of natural killer cell lines from murine long-term bone marrow cultures. J Immunol. 1985 Mar;134(3):1462–1468. [PubMed] [Google Scholar]


