Abstract
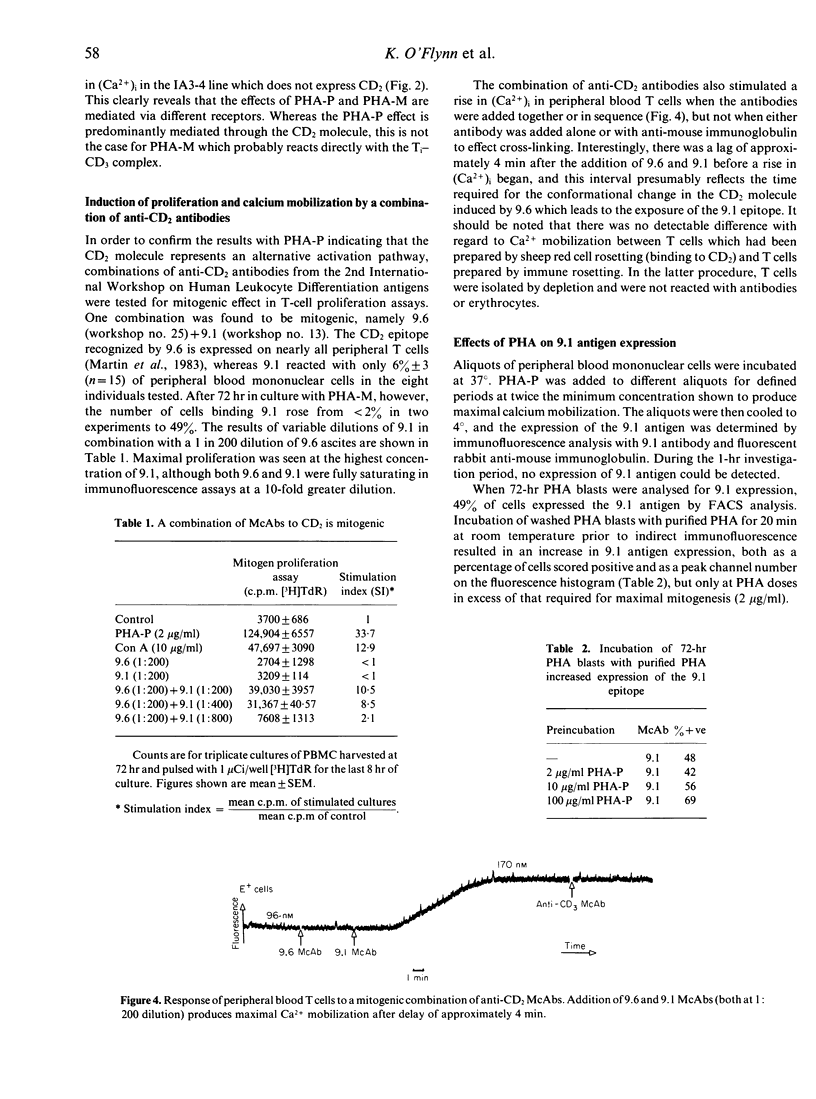
Antigen-specific T-cell activation is mediated via the CD3-Ti (antigen receptor) complex, and monoclonal antibodies to both CD3 and Ti cause a rapid rise in intracellular Ca2+. This calcium mobilization is not inhibited by monoclonal antibodies to CD2. The rise in calcium mobilization induced by purified PHA (PHA-P) does not occur in a cell line which lacks CD2 expression, and can be blocked in other T cells by anti-CD2 antibodies. A combination of monoclonal antibodies to different epitopes of CD2 causes calcium mobilization and mitogenesis. Reagent grade PHA (PHA-M) induces calcium moblization in cells that lack CD2, and its effects in other T cells cannot be blocked by anti-CD2 antibodies. The effects of PHA-P and PHA-M are thus mediated predominantly through different activation pathways.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando I., Hariri G., Wallace D., Beverley P. Tumor promoter phorbol esters induce unresponsiveness to antigen and expression of interleukin 2 receptor on T cells. Eur J Immunol. 1985 Feb;15(2):196–199. doi: 10.1002/eji.1830150217. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Boylston A. W., Goldin R. D., Moore C. S. A human T cell tumor which expresses the putative T cell antigen receptor. Eur J Immunol. 1984 Mar;14(3):273–275. doi: 10.1002/eji.1830140313. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kanellopoulos J. M., De Petris S., Leca G., Crumpton M. J. The mitogenic lectin from Phaseolus vulgaris does not recognize the T3 antigen of human T lymphocytes. Eur J Immunol. 1985 May;15(5):479–486. doi: 10.1002/eji.1830150512. [DOI] [PubMed] [Google Scholar]
- Lichtman A. H., Segel G. B., Lichtman M. A. Calcium transport and calcium-ATPase activity in human lymphocyte plasma membrane vesicles. J Biol Chem. 1981 Jun 25;256(12):6148–6154. [PubMed] [Google Scholar]
- Martin P. J., Longton G., Ledbetter J. A., Newman W., Braun M. P., Beatty P. G., Hansen J. A. Identification and functional characterization of two distinct epitopes on the human T cell surface protein Tp50. J Immunol. 1983 Jul;131(1):180–185. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Minowada J., Janossy G., Greaves M. F., Tsubota T., Srivastava B. I., Morikawa S., Tatsumi E. Expression of an antigen associated with acute lymphoblastic leukemia in human leukemia-lymphoma cell lines. J Natl Cancer Inst. 1978 Jun;60(6):1269–1277. doi: 10.1093/jnci/60.6.1269. [DOI] [PubMed] [Google Scholar]
- O'Flynn K., Krensky A. M., Beverley P. C., Burakoff S. J., Linch D. C. Phytohaemagglutinin activation of T cells through the sheep red blood cell receptor. Nature. 1985 Feb 21;313(6004):686–687. doi: 10.1038/313686a0. [DOI] [PubMed] [Google Scholar]
- O'Flynn K., Linch D. C., Tatham P. E. The effect of mitogenic lectins and monoclonal antibodies on intracellular free calcium concentration in human T-lymphocytes. Biochem J. 1984 Apr 15;219(2):661–666. doi: 10.1042/bj2190661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flynn K., Zanders E. D., Lamb J. R., Beverley P. C., Wallace D. L., Tatham P. E., Tax W. J., Linch D. C. Investigation of early T cell activation: analysis of the effect of specific antigen, interleukin 2 and monoclonal antibodies on intracellular free calcium concentration. Eur J Immunol. 1985 Jan;15(1):7–11. doi: 10.1002/eji.1830150103. [DOI] [PubMed] [Google Scholar]
- Oettgen H. C., Terhorst C., Cantley L. C., Rosoff P. M. Stimulation of the T3-T cell receptor complex induces a membrane-potential-sensitive calcium influx. Cell. 1985 Mar;40(3):583–590. doi: 10.1016/0092-8674(85)90206-5. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Daley J. F., Hodgdon J. C., Reinherz E. L. Calcium dependency of antigen-specific (T3-Ti) and alternative (T11) pathways of human T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6836–6840. doi: 10.1073/pnas.81.21.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]



