Abstract
Oropharyngeal candidiasis (OPC), caused by Candida albicans, is the most frequent opportunistic fungal infection in human immunodeficiency virus (HIV)-positive persons. Although Th1-type CD4+ T cells are considered important for host defense against mucosal C. albicans infections, there is a paucity of information regarding the presence and/or role of T cells in OPC lesions. In pursuit of this, initial chromophore immunohistochemical studies showed a majority of CD8+ rather than CD4+ cells equally distributed throughout the buccal mucosa of OPC− persons (HIV− or HIV+), irrespective of blood CD4+ cell numbers. In contrast, CD8+ cells in lesions from HIV+ OPC+ persons were in significantly higher numbers and concentrated at the lamina propria-epithelium interface, a considerable distance from the Candida at the outer epithelium. Dual fluorescence and confocal microscopy confirmed that the majority of CD8+, but not CD4+, cells were T cells by the presence or absence, respectively, of CD3 on each cell type. These results suggest that CD8+ T cells may be important for oral host defense against OPC, especially when CD4 cell numbers are reduced, with a potential CD8 cell-specific dysfunction associated with susceptibility to OPC.
Oropharyngeal candidiasis (OPC), caused by Candida albicans, is a common oral opportunistic infection in those with the human immunodeficiency virus (HIV) (12, 14, 16, 20). C. albicans is a ubiquitous fungal organism that is part of the normal microflora of the gastrointestinal and reproductive tracts (6). Due to exposure to C. albicans early in life, most healthy individuals have anti-Candida immunity that protects against infection. However, under immunocompromised conditions, (i.e., HIV infection, transplantation, corticosteroid therapy, and lymphoma) C. albicans is capable of rapid conversion to a pathogen causing symptomatic mucosal infections (2, 5, 14, 15, 20, 22). Clinically, OPC can be observed in lesions as a mixture of hyphae and yeast, typically residing in the stratum corneum-keratin layer of the outer epithelium, and involves infections of the buccal mucosa (BM), gingival cuff, palate, retromolar pad, alveolar ridges, and tongue. The infections can be erythematous, atrophic lesions that appear reddish, or pseudomembranous, presenting as white curd-like lesions (thrush) (4). OPC can lead to difficulty chewing and painful swallowing, ultimately leading to decreased nutritional intake with significant morbidity (9).
Cell-mediated immunity (CMI) by Th1-type CD4+ T cells is considered the most important host defense mechanism against C. albicans at mucosal surfaces as demonstrated by the high incidence of mucosal candidiasis in those with reduced CD4+ T cells (8, 12-14, 17, 22, 23, 25, 28). However, recent studies show little evidence for a deficiency in Candida-specific systemic CMI in those with OPC (17). In studies conducted on local immune mechanisms, Candida-specific antibodies in saliva were found to be similar in those with or without OPC, suggesting little to no role for humoral immunity in resistance or susceptibility to OPC (33). In contrast, individuals with OPC had reduced anti-Candida activity by oral epithelial cells (30) and a shift in the Th cytokines in saliva from Th1- to Th2-type (18), the latter finding suggesting some role for local CMI in susceptibility to OPC.
A limited number of formal surveys of T lymphocytes in oral lesions of HIV-infected persons with OPC have been reported (21, 27, 32). Although CD4+ and CD8+ T cells have been detected in OPC lesions, no studies to date have correlated CD3+ T cells with CD4+ and CD8+ cells and critically evaluated the cells in relation to the organism. Additionally, strict stratification of patients with regard to blood CD4+ T cells has not been conducted.
The purpose of the present study was to conduct a comprehensive survey of the oral T lymphocyte profile (CD4+ and CD8+) from an established cohort of HIV+ OPC+ (including infected and uninfected sites), HIV+ OPC−, and HIV-uninfected individuals by chromophore and fluorescent immunohistochemical staining.
MATERIALS AND METHODS
Subjects.
Patients were recruited and evaluated at the Louisiana State University (LSU) Health Sciences Center HIV Outpatient Dental Clinic associated with the HIV Outpatient Program of the Medical Center of Louisiana at New Orleans and the Charity Hospital Dental Clinic. Informed consent was obtained from all participants and/or patients, and all procedures in the conduct of clinical research were done in accordance with the Institutional Review Board at the LSU Health Sciences Center, New Orleans. Subjects were part of an established cohort (n = 239) comprised of 86 HIV-uninfected persons and 153 HIV-infected persons, including 68 HIV+ OPC+ and 85 HIV+ OPC− persons. A subset of the larger cohort was used for the immunohistochemical analyses, including HIV− (n = 6), HIV+ OPC− (n = 14), and HIV+ OPC+ (n = 19) persons. Of these, 9 OPC− and 15 OPC+ individuals had <200 CD4 cells/μl. Seventy-three percent of the HIV+ persons in the subset were receiving highly active antiretroviral therapy (HAART). In this cohort, HAART was defined as three or more antiretroviral medications, whereas monotherapy or dual therapy without a protease inhibitor was defined as non-HAART.
Diagnosis of oropharyngeal candidiasis and detection of oral yeast colonization.
The diagnosis of OPC was made on the clinical appearance of red, atrophic areas (erythematous) or white curd-like plaques (pseuodomembranous) on the oral mucosa (29). Pseudomembranous and erythematous infections composed 89 and 11%, respectively, of those with OPC. Oral swabs from both infected and uninfected regions of the BM were plated on Sabouraud-dextrose agar (SAB; Becton Dickinson Microbiology Systems, Franklin, N.J.) and Chromagar (CHROMagar Microbiology, Paris, France) and incubated for 48 h at 34 and 37°C, respectively. Identification of OPC was further confirmed by hyphae present on a wet mount slide preparation by using potassium hydroxide (KOH) and a positive swab culture with characteristic colony morphology. Initial speciation was screened for by color on Chromagar. Green colonies were processed for germ tube formation (incubation in fetal bovine serum [FBS] for 2 h at 37°C), with those forming germ tubes identified as C. albicans. Nongreen colonies were speciated by API biochemical tests (API 20 AUX; BioMerieux, Durham, N.C.).
Within the subcohort, ∼50% of HIV− individuals were asymptomatically colonized with yeast. Of these, ∼90% were colonized with C. albicans. Of the HIV+ OPC− patients, ∼80% had detectable asymptomatic yeast colonization. Of these patients, ∼85% were colonized with C. albicans. For the OPC+ patients, only those with C. albicans pseudomembranous OPC (represented by 90% of those with OPC in the entire cohort) were included in the immunohistochemical analysis.
Sample collection or processing. (i) Blood.
Venous blood (10 ml) was collected from each subject. HIV status was verified in serum by enzyme-linked immunosorbent assay, followed by confirmatory Western blot by the Clinical Immunology Laboratory at the LSU Health Sciences Center. CD4 and CD8 lymphocyte counts were quantified by flow cytometry.
(ii) Biopsy.
Elliptical biopsies were taken from the BM of HIV− and HIV+ OPC− individuals and from both infected and noninfected regions of HIV+ OPC+ persons. BM was excised from the oral cavity and oriented for cross-sectional analysis in Tissue-Tek cryomolds (Miles Corp, Elkhart, Ind.), by using optimum cutting temperature (OCT) medium (Sakura Finetek USA, Inc., Torrance, Calif.). Tissue was snap-frozen and stored at −70°C. Frozen tissue was sectioned (6 μm), collected on glass slides, and fixed in 3% formaldehyde (PolyLEM; Polysciences, Inc., Warrington, Pa.) for 2 min, followed by ice-cold acetone (5 min) and stored at −20°C.
Immunohistochemistry. (i) Hematoxylin and eosin.
BM tissue sections were stained with hematoxylin and eosin (Biochemical Sciences, Inc., Swedesboro, N.J.) according to the manufacturer's instructions in order to confirm tissue orientation.
(ii) Chromophore staining of cell surface antigen.
Serial sections of BM biopsies were rehydrated in phosphate-buffered saline (PBS) for 5 min. The endogenous peroxidase activity was blocked by incubating the sections in 3% hydrogen peroxide (peroxidase blocking reagent; R&D Systems, Minneapolis, Minn.) for 2 min. After a wash in PBS, nonspecific protein-binding sites in the tissue were blocked by incubating in normal mouse serum (R&D Systems) for 15 min. This was followed by the addition of two additional blocking reagents, including avidin-0.1% sodium azide (avidin blocking reagent) (R&D Systems), a wash in PBS, and then biotin-0.1% sodium azide (biotin blocking reagent) (R&D Systems) (each for 10 min). The sections were washed in PBS and incubated overnight at 37°C with monoclonal mouse anti-human CD3, CD4, or CD8 antibodies (10 μg/ml; Dako Corp., Cambridge, Mass.) in a humidified chamber. Negative controls consisted of tissue incubated with isotype-specific, purified mouse immunoglobulin (Dako Corp.). After overnight incubations, the slides were washed in PBS and incubated with a biotinylated goat anti-mouse immunoglobulin G (IgG; 10 μg/ml; R&D Systems) for 2 h. Thereafter, the sections were washed and incubated for 30 min with high-sensitivity streptavidin-horseradish peroxidase conjugate (R&D Systems). To washed sections, the substrate 3-amino-9-ethylcarbazole chromogen (R&D Systems) was added for 5 to 10 min. Mayer's hematoxylin (Fisher Diagnostics, Fair Lawn, N.J.) was used as the counterstain. Slides were preserved by using Crystal Mount aqueous mounting solution (Biomedia, Foster City, Calif.).
(iii) Immunofluorescent staining of cell surface antigen.
BM sections were rehydrated in PBS. Nonspecific protein binding sites were blocked with bovine serum albumin (1%) and 5% goat serum (blocking reagent). The slides were washed in PBS and the sections were incubated for 2 h at 25°C with monoclonal rat anti-human CD3 (10 μg/ml; Vector Laboratories, Burlingame, Calif.) in a humidifier. The sections were washed in PBS and incubated with biotinylated anti-rat IgG (10 μg/ml; secondary antibody; Vector Laboratories) for 1 h. Washed sections were incubated with fluorescein avidin D (Vector Laboratories) for 30 min. The sections were then washed, and the blocking reagent was added a second time for 15 min, followed by a wash and the addition of monoclonal mouse anti-human CD4 or CD8 (10 μg/ml; Vector Laboratories) (depending on the desired T-cell marker) for 1 h at 25°C. After being washed, the sections were incubated with biotinylated anti-mouse IgG (10 μg/ml; Vector Laboratories) for 1 h at 25°C, washed, and incubated with Texas red avidin D (Vector Laboratories) for 30 min. Controls included isotype-specific antibody (10 μg; Vector Laboratories) after incubation with anti-CD3 antibody (control for second antibody system stain) or isotype antibody, followed by the addition of either anti-CD4 or anti-CD8 antibody (control for first antibody system staining). All other steps were conducted as outlined above. Using a fluorescent microscope (Nikon, Tokyo, Japan), along with accompanying MetaView software (Universal Imaging Corp., Downingtown, Pa.), the image from the CD3-labeled tissue section (fluorescein avidin D) was acquired by using an fluorescein isothiocyanate (FITC) filter. The images from the CD4- and CD8-labeled tissue sections (Texas red avidin D) were acquired by using a TRITC (tetramethyl rhodamine isothiocyanate) filter. The two acquired images were then overlaid. Cells containing both FITC and TRITC labels appear yellow.
Tissue-associated fungal organism identification.
BM sections were placed in periodic acid solution (1 g/dl in deionized water) for 2 min and washed in five to six changes of water. Sections were placed into a silver methenamine solution and incubated for 20 min in a 62°C water bath. The sections were then washed in 62°C water, followed by four additional washes in water at room temperature. Sections were counterstained with Mayer's hematoxylin. Hyphae were observed microscopically.
Tissue-associated lymphocyte analysis.
By using bright-field microscopy, three defined areas (∼37,000 μm2/each) were determined to be representative of the lamina propria-epithelial border under investigation. These areas of the tissue section were gated and the stained T cells were marked (“painted”) by using MetaView software (Universal Imaging Corp.). A percent threshold of tissue-associated T cells was then quantified inside the unit area. This same area was then used to quantify the numbers of cells in uninfected tissue sections from the same patient and in sections from HIV− and HIV+ OPC− persons. For correlative analyses of CD8+ cells, the numbers of stained cells in a given area were given a score from 1 (low) to 6 (high) for each individual (1 = average of <10 cells/field, 2 = up to 30 cells/field, 3 = up to 50 cells/field, 4 = up to 75 cells/field, 5 = up to 100 cells/field, 6 = >100 cells/field) at ×400 magnification with up to five fields examined and expressed as a score in 0.1 increments.
Statistics.
Differences in percent threshold CD8 were identified by using the Mann-Whitney U test. Differences in blood CD4 or CD8 cells or fungal burden were identified by using the Student's t test. Significant differences were defined as P < 0.05 by using a two-tailed test. A coefficient of determination (r2) was used to correlate the fungal burden and tissue-associated CD8+ T cells. All statistics were performed by using GraphPad Prism (GraphPad Software, San Diego, Calif.).
RESULTS
Immunohistochemical analysis of BM in OPC− lesions.
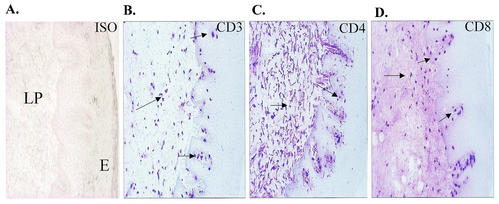
In order to examine T-cell populations at the oral mucosa in those with OPC, we first aimed to gather baseline data in OPC− persons with characteristics similar to those with OPC (<200 CD4+ cells/μl). For this, tissue biopsies were taken from the BM of HIV+ OPC− persons with <200 CD4+ cells/μl, and serial tissue sections were immunohistochemically stained with anti-CD3, CD4, and CD8 antibodies. Figure 1 shows representative results from serial BM sections. Isotype controls showed no appreciable staining (panel A). CD3+ (panel B) and CD8+ (panel D) cells were present in both the epithelium and lamina propria with the majority of cells in the lamina propria. CD4+ cells (panel C) were more numerous and also primarily present in the lamina propria. CD3+ cells matched more to the CD8+ cells than to the CD4+ cells, suggesting that the majority of CD3+ cells were CD8+. The results were similar in those with ≥200 CD4 cells/μl (data not shown).
FIG. 1.
Distribution of T cells in BM of HIV+ OPC− individuals with <200 cells/μl. Serial BM tissue sections were immunohistochemically stained for CD3+, CD4+, and CD8+ cells. The figure shows a representative chromophore staining of BM tissues with isotype control antibodies (A), anti-CD3 antibodies (B), anti-CD4 antibodies (C), and anti-CD8 antibodies (D) from among 14 subjects examined. Arrows point to positively stained cells. Magnification, ×70. LP, lamina propria; E, epithelium.
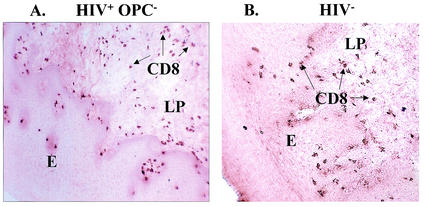
We next asked whether there were any distinguishable differences in the CD8+ T-cell distribution in HIV+ OPC− individuals and in HIV− individuals. For this, BM sections from HIV+ OPC− and HIV− persons were immunohistochemically evaluated. As seen in the representative image in Fig. 2, an equal distribution of CD8+ T cells was evident throughout the lamina propria and epithelium of both the HIV+ OPC− (panel A) and the HIV− individuals (panel B). The results did not differ whether the HIV+ OPC− persons had <200 or ≥200 CD4 cells/μl (results for the HIV+ OPC− individual with <200 CD4 cells/μl is shown).
FIG. 2.
Distribution of CD8+ cells in HIV+ OPC− and HIV− individuals. BM tissue sections were chromophore stained for CD8+ cells. The figure shows representative images of CD8 T-cell distributions from among 14 HIV+ OPC− individuals (A) and 6 HIV− individuals (B). The HIV+ OPC− image shown is from a person with <200 CD4 cells/μl. The arrows point to CD8+ cells. Magnification, ×70. LP, lamina propria; E, epithelium.
Immunohistochemical analysis of BM in OPC+ lesions.
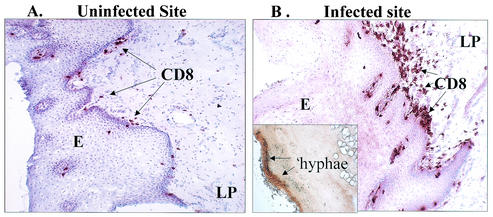
We next evaluated the CD3+ and CD8+ T cells in HIV+ OPC+ individuals. For this, infected and uninfected BM biopsies from those with pseudomembranous OPC were collected, sectioned, and immunohistochemically stained. A representative image of an individual with <200 CD4 cells/μl is shown in Fig. 3. The uninfected site (panel A) showed the characteristic distribution of CD8+ cells similar to OPC− individuals. In contrast, the infected site (panel B) showed an accumulation of CD8+ cells at the lamina propria-epithelium interface at a considerable distance from the location of the hyphae that were present at the outer epithelium (insert shows silver stain for Candida hyphae). CD4+ cells were equally present in large numbers in both infected and uninfected sites without discernible differences (data not shown).
FIG. 3.
CD8+ T-cell distribution in BM tissues from uninfected and infected sites of HIV+ OPC+ individuals. The tissue sections were stained for CD8+ cells. The figure shows representative images of an uninfected site (A) and an infected site (B) from among 19 HIV+ OPC+ patients examined. A silver stain for Candida hyphae at the outer epithelium is shown in the insert. The arrows point to CD8+ cells identified. Magnification, ×70. LP, lamina propria; E, epithelium.
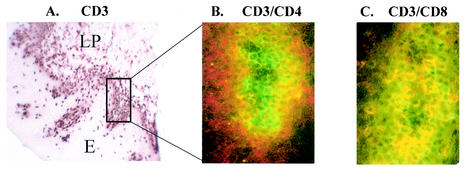
In order to confirm that the CD8+, but not CD4+, cells shown in the chromophore-labeled serial sections were indeed T cells, tissue sections from similar individuals (HIV+ OPC+, <200 CD4 cells/μl) were stained with the chromophore system, whereas other sections were dually stained with anti-CD3 antibodies conjugated to fluorescein avidin D (FITC/green) and with anti-CD4 or anti-CD8 antibodies conjugated to Texas red avidin D (TR/red), and then analyzed by fluorescence confocal microscopy. A representative illustration of the chromophore staining for CD3 and the confocal overlay for CD3/CD4 and CD3/CD8 is shown in Fig. 4. The chromophore section shows the accumulation of CD3+ cells (panel A) and the region highlighted (boxed frame) for the fluorescent images. Of the confocal images (panels B and C), the majority of the double-stained (yellow) cells occurred in the CD3/CD8 dual stain (panel C) compared to the CD3/CD4 dual stain (panel B). In fact, the CD3+ (green) and CD4+ (red) cells are clearly present in different areas, indicating separate cell populations. Isotype controls for both antibody systems (fluorescein avidin D and Texas red avidin D) showed negligible staining for each isotype used with no double-stained cells observed, and dual-stained images examined with either of the single fluorescent filters showed positively stained cells as expected (data not shown). Similar chromophore and fluorescence results were observed in those with OPC and ≥200 CD4 cells/μl (data not shown).
FIG. 4.
Chromophore and dual fluorescence staining of T cells in the BM of an HIV+ OPC+ individual with <200 cells/μl. (A) Chromophore staining for CD3. The boxed frame shows the area of tissue that the fluorescence images represent. (B) Confocalized fluorescence image of CD3+/CD4+ T cells. (C) Confocalized fluorescence image of CD3+/CD8+ T cells. E, epithelium; LP, lamina propria. Magnifications: ×90 (chromophore image), ×360 (fluorescence images).
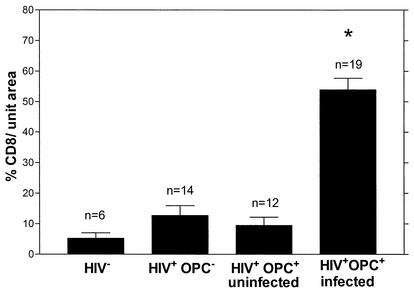
Quantitative analysis of CD8+ cells for BM sections from HIV− (n = 6) and HIV+ OPC− (n = 14) individuals and for both noninfected (n = 12) and infected (n = 17) sites of HIV+ OPC+ individuals was subsequently conducted. The results in Fig. 5 show a significant increase (P < 0.0004) in the numbers of CD8+ T cells in the infected sites of the HIV+ OPC+ group compared to all other groups analyzed. Table 1 summarizes the oral fungal burden and blood CD4 and CD8 cell numbers in the subcohort evaluated. The mean CD4+ cell number for all OPC+ and OPC− individuals was significantly different, whereas the mean CD8+ cell numbers for the same individuals was not. The mean fungal burden associated with the BM for those with OPC (lesion) was significantly higher than in those without OPC (sampling of tissue with asymptomatic colonization).
FIG. 5.
Quantitative analysis of CD8+ cells in BM tissue. The percent threshold values of CD8+ cells in oral sites were quantified per unit area (∼37,000 μm2) for HIV− individuals (n = 6), HIV+ OPC− individuals (n = 14), and for both uninfected (n = 12) and infected sites (n = 19) of HIV+ OPC+ individuals. The asterisk indicates a significant difference compared to all other groups analyzed (P < 0.0004).
TABLE 1.
Investigative cohort of HIV+ OPC+ and HIV+ OPC− individuals
| Parameter | HIV+ OPC+ (n = 19) | HIV+ OPC− (n = 14) | P |
|---|---|---|---|
| Mean fungal burdena (CFU) ± SEM | 810 ± 74 | 190 ± 95 | <0.0002 |
| Mean no. of CD4+ cells/μl of blood ± SEM | 100 ± 36 | 290 ± 100 | <0.0166 |
| Mean no. of CD8+ cells/μl of blood ± SEM | 550 ± 100 | 530 ± 100 | NSb |
| No. of individuals with < 200 CD4+ cells/μl of blood | 15 | 9 | |
| No. of individuals with ≥200 CD4+ cells/μl of blood | 4 | 5 |
Derived from swab culture of buccal mucosa. For OPC+ persons, a swab was taken from the lesion under investigation. For OPC− persons, a swab was taken from several areas of the BM.
NS, not significant (P > 0.05).
Correlation between fungal burden and tissue-associated CD8+ T cells in OPC− and OPC+ persons.
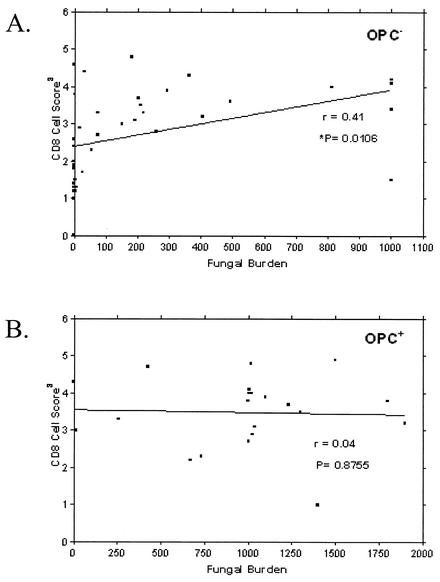
Finally, we asked whether the presence of CD8+ cells in BM correlated to oral fungal burden. For this, CD8+ T-cell numbers were given a score from 1 (low) to 6 (high) for each individual and compared to the numbers of CFU identified from oral swabs. Figure 6A reveals a positive correlation between the numbers of CD8+ T cells and oral fungal burden in HIV+ OPC− persons (P = 0.0106). In contrast, no correlation was observed between numbers of CD8+ cells and fungal burden in HIV+ OPC+ persons (Fig. 6B).
FIG. 6.
Correlation between fungal burden and tissue-associated CD8+ cells in OPC− and OPC+ individuals. CD8+ T cells in BM tissue were given a score from 1 (low) to 6 (high) for each individual and compared to oral fungal burden in terms of CFU. (A) OPC− individuals; (B), OPC+ individuals. Correlation coefficients (r) and P values are shown. Significance was defined as P < 0.05.
DISCUSSION
OPC continues to persist in HIV+ individuals, despite the use of HAART (24, 26). Although the occurrence of OPC correlates to reduced CD4+ T-cell numbers in blood (<200 CD4 cells/μl) (8, 12-14, 17, 22, 23, 25, 28), a recent study that evaluated Candida-specific systemic CMI in HIV+ persons with or without OPC showed no overt deficiency in peripheral blood lymphocyte responsiveness (17). It was therefore postulated that a threshold number of blood-derived CD4+ T cells were required to protect against OPC. Below the threshold (usually <200 CD4 cells/μl), local immune mechanisms are considered important for protection. Accordingly, a profound Th2-type cytokine pattern present in saliva of HIV-infected patients with OPC (particularly in individuals with <200 CD4 cells/μl) (18) is consistent with the general susceptibility to mucosal candidiasis. Additionally, in vitro anti-Candida activity by oral epithelial cells (collected from saliva) (30) was found to be reduced in those with OPC (30). In contrast, a recent comprehensive analysis of Candida-specific antibodies in those with OPC, stratified by CD4+ cell numbers, showed no demonstrable deficiency in humoral immunity associated with susceptibility to OPC. On the other hand, analyses of local T cells have been sparse in those with OPC (21, 27, 32). Such analyses, however, are critical to a better understanding of the deficient host response associated with susceptibility to OPC.
In pursuit of this, the presence of CD4+ and CD8+ T cells in individuals with OPC was evaluated in significant detail. Interestingly, chromophore staining of serial sections of BM in HIV+ OPC− persons, irrespective of blood CD4 cell number, showed that the majority of CD3+ T cells matched more closely with CD8+ cells, not CD4+ cells. This observation was true as well for HIV− persons. The high numbers of CD3− CD4+ cells identified in the tissue sections were largely irregular in shape, supporting the contention that the majority of CD4+ cells present were not T cells. We postulate that the CD3− CD4+ cells are macrophages or dendritic cells that express CD4 (11). Immunohistochemical staining for macrophage/dendritic cells will be required to confirm this hypothesis. Even though the distribution of CD4+ cells in the tissue was similar and numerous in HIV+ OPC− and HIV− individuals, our data suggest that numbers of CD4+ T cells are extremely low in the tissue of individuals with either ≥200 or <200 blood CD4+ T cells. The low levels of double-stained cells seen after dual staining for both CD3 and CD4 of OPC− persons (data not shown) support this. We do not exclude the possibility, however, that CD4+ T cells may be found in somewhat higher numbers in individuals with ≥200 blood CD4+ cells but may be masked by the presence of large numbers of CD3− CD4+ cells. In any event, low numbers of tissue-associated CD4+ T cells is reasonable based on the fact that blood-derived CD4+ T cells are most likely only present when specifically recruited from the peripheral circulation in response to high numbers of organisms or preacute infection. The probability of observing this condition in the random enrollment of any OPC− patient would be considered quite low, much less the probability of choosing a specific biopsy site that would be affected.
In BM lesions from HIV+ individuals with pseudomembranous OPC, we observed an accumulation of CD8+ T cells at the lamina propria-submucosal interface at a considerable distance from the superficial site of Candida infection at the outer epithelium compared to an uninfected site within the same patient. These CD8+ cells were confirmed to be T cells by dual fluorescence staining and confocal microscopy with anti-CD3 and anti-CD8 antibodies where the majority of CD3+ cells were also CD8+. In fact, quantitative analysis showed significantly higher numbers of CD8+ cells in OPC lesions compared to uninfected tissue of the same patient or in tissue from HIV+ OPC− patients or HIV− persons. In contrast, CD4+ cells were similarly distributed in OPC+ lesions compared to an uninfected site or BM from OPC− persons, and dual fluorescent staining for CD3 and CD4 confirmed that the majority of these CD4+ cells were CD3− and thus not T cells. The lack of tissue-associated CD4+ T cells in those with OPC is consistent with the reduced levels of blood CD4+ T cells. Interestingly, the increased numbers of CD8+ T cells in infected lesions did not differ regardless of whether the patient had <200 or ≥200 CD4 cells/μl or whether the patient had infrequent or recurrent episodes of OPC (data not shown). Moreover, numbers of CD8+ T cells in peripheral blood were not significantly different between OPC+ and OPC− persons, suggesting that the increased presence of CD8+ T cells at the site of infection was not influenced by differences in the systemic levels of CD8+ T cells. Interestingly, there was also little evidence for the presence of neutrophils in the lesions or microabscesses as shown by H&E staining (data not shown).
The presence of CD8+ T cells in the tissue is consistent with some limited immunohistochemical analysis showing the presence of CD4+ and CD8+ cells in lesions of those with OPC and others that reported the presence of CD8+ cells exclusively (21, 27, 32). However, the previous studies did not quantify the levels of CD8+ or CD4+ cells in the lesions or assess the location of the cells relative to the location of Candida. Furthermore, infected and uninfected sites were not evaluated in the same patient, nor was dual fluorescence performed to confirm that the CD4+ and CD8+ cells were T cells. Thus, the present study represents a definitive analysis of local T lymphocytes during OPC. What is unclear, however, is whether the CD8+ cells are of local origin or infiltrated from the peripheral circulation. The predominance of CD8+ cells rather than CD4+ cells under OPC− conditions and the lack of correlation to numbers of blood CD8+ cells suggests a local origin with increased numbers in the tissue by proliferation. In any event, the cells are indeed present locally, a finding suggestive of some role as a host defense mechanism against infection.
Based on our data, we postulate that although blood-associated CD4+ T cells play a primary role in host defense against OPC through infiltration when recruited in response to higher numbers of Candida or preacute infection in the oral cavity, CD8+ T cells play a putative role in protection against OPC in those colonized with Candida and assume a primary defense role when blood CD4+ T cells are reduced below the protective threshold. It should be noted that the actual protective threshold level of CD4 cells varies among individuals. For example, although 200 CD4 cells/μl is the threshold number most often referred to, smokers often acquire OPC when CD4 cell levels drop to <500 cells/μl (29). In any event, we postulate that, under normal circumstances, CD8+ T cells can migrate effectively to the outer epithelium as part of the normal host response and aid in defense against infection along with recruited CD4+ T cells (when necessary) and antifungal activity by oral epithelial cells. This view is supported by the positive correlation of CD8+ T-cell numbers in the oral mucosa to the fungal burden during detectable asymptomatic colonization in OPC− individuals. Additionally, we noted that the CD8+ T cells in the BM of OPC− individuals were evenly distributed throughout the tissue, including areas near the outer epithelium. If this hypothesis is correct, and especially under conditions of reduced CD4+ T cells, we contend that susceptibility to OPC may result from a dysfunction in the migration of CD8+ T cells to the outer epithelium, resulting in the accumulation of CD8+ T cells at a considerable distance from the site of infection. However, the level of fungal burden in OPC+ individuals does not appear to influence the numbers of CD8+ cells accumulated based on correlative analyses. Irrespective of this, it is interesting to speculate that the accumulation of these CD8+ T cells may be responsible for the edema observed at the lesion site. It is unclear what is responsible for the putative CD8 T-cell dysfunction and whether Candida itself plays a role at a certain threshold. HIV is another likely candidate if we take into account the higher frequency of OPC in HIV-infected persons compared to other groups with reduced CD4+ T cells (i.e., transplantation recipients and lymphoma patients on chemotherapy [clinical observations]). Alternative explanations for the presence of CD8+ T cells involves the cells acting as a barrier against dissemination of hyphae into the peripheral circulation as part of a normal protective response against candidemia or an adequate activity of the CD8+ cells within the confines of the area observed leaving the infection isolated to areas not easily accessible by the cells (outer epithelium).
Historically, CD8+ T cells have not been considered critical as a protective host defense against Candida. This was probably due to the predominant role for CD4+ T cells against candidal infections, the lack of studies focusing on a protective role of CD8+ T cells, and the general acceptance that CD8+ T cells were more critical for suppression or downregulation of CD4+ T-cell responses (7, 10). Nevertheless, our data revealing a putative role for CD8+ T cells against Candida are supported by both experimental and clinical data from Mathews and coworkers (1, 3). Experimentally, CD8+ T cells were shown to inhibit the growth of Candida in vitro in a non-major histocompatibility complex-restricted manner (1). Clinically, a similar activity was observed with peripheral blood lymphocytes from HIV-positive persons who had had a recent episode of OPC (3). Thus, there are multiple lines of evidence supporting a role for CD8+ T cells in host defense against Candida infection. These nonconventional activities of CD8+ T cells are also similar in principle to the anti-HIV CD8+ T cells described by Levy and coworkers (19, 31) and may in fact be present as a result of HIV, especially if these cells function in a non-major histocompatibility complex-restricted manner. This view is plausible, since there are no reports of antigen-specific cytotoxic activity of CD8+ T cells (i.e., cytotoxic T lymphocytes) against Candida.
In summary, based on the pattern of CD8+ T-cell accumulation seen in OPC+ lesions, our data suggest some role for CD8+ T cells in host defense against OPC. Although the mechanism remains unclear, we propose that a dysfunction in this presumed CD8+ T-cell activity against Candida may enhance susceptibility to OPC, especially in individuals with CD4+ T cells below a protective threshold. Confirmation of this hypothesis will require the analysis of activation markers, homing receptors, and chemokine receptors on the CD8+ T cells in those with or without OPC.
Acknowledgments
This work was supported by a National Institutes of Health Public Health Service grant DE 12178 from the National Institute of Dental and Craniofacial Research and by the Louisiana Board of Regents through the Millenium Trust Health Excellence Fund [HEF (2000-05)-04].
Editor: T. R. Kozel
REFERENCES
- 1.Beno, D. W. A., A. G. Stover, and H. L. Mathews. 1995. Growth inhibition of Candida albicans hyphae by CD8+ lymphocytes. J. Immunol. 154:5273-5281. [PubMed] [Google Scholar]
- 2.Clift, R. A. 1984. Candidiasis in the transplant patient. Am. J. Med. 77(Suppl. 4D):34-38. [PubMed] [Google Scholar]
- 3.Colon, M., N. Toledo, C. L. Valiente, N. Rodriquez, N. Yano, and H. L. Mathews. 1998. Anti-fungal and cytokine producing activities of CD8+ T lymphocytes from HIV-1-infected individuals. AIDS Res. 90:21-26. [PubMed] [Google Scholar]
- 4.Dodd, C. L., D. Greenspan, M. H. Katz, J. L. Westenhouse, D. W. Feigal, and J. S. Greenspan. 1991. Oral candidiasis in HIV infection: pseudomembranous and erythematous candidiasis show similar rates of progression to AIDS. AIDS 5:1339-1343. [PubMed] [Google Scholar]
- 5.Fichtenbaum, C. J., and W. Powderly. 1998. Refratory mucosal candidiasis in patients with human immunodeficiency virus infection. Clin. Infect. Dis. 26:556-565. [DOI] [PubMed] [Google Scholar]
- 6.Fidel, P. L., Jr. 1999. Host defense against oropharyngeal and vaginal candidiasis: site-specific differences. Rev. Iberoam. Micol. 16:8-15. [PubMed] [Google Scholar]
- 7.Fidel, P. L., Jr., M. E. Lynch, and J. D. Sobel. 1994. Effects of preinduced Candida-specific systemic cell-mediated immunity on experimental vaginal candidiasis. Infect. Immun. 62:1032-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer, A., J. J. Ballet, and C. Griscelli. 1978. Specific inhibition of in vitro Candida-induced lymphocyte proliferation by polysaccharide antigens present in serum of patients with chronic mucocutaneous candidiasis. J. Clin. Investig. 62:1005-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher-Hoch, S. P., and L. Hutwagner. 1995. Opportunistic candidiasis: an epidemic of the 1980s. Clin. Infect. Dis. 21:897-904. [DOI] [PubMed] [Google Scholar]
- 10.Garner, R. E., A. M. Childress, L. G. Human, and J. E. Domer. 1990. Characterization of Candida albicans mannan-induced, mannan-specific delayed hypersensitivity suppressor cells. Infect. Immun. 58:2613-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grabbe, S., E. Kampgen, and G. Schuler. 2000. Dentritic cells: multi-lineal and multi-functional. Immunol. Today 21:431-433. [DOI] [PubMed] [Google Scholar]
- 12.Greenspan, J. S., C. E. Barr, J. J. Sciubba, and J. R. Winkler. 1992. Oral manifestations of HIV infection: definitions, diagnostic criteria and principles of therapy. Oral Surg. Oral Med. Oral Pathol. 73:142-144. [DOI] [PubMed] [Google Scholar]
- 13.Imam, N., C. C. J. Carpenter, K. H. Mayer, A. Fisher, M. Stein, and S. B. Danforth. 1990. Hierarchical pattern of mucosal Candida infections in HIV-seropositive women. Am. J. Med. 89:142-146. [DOI] [PubMed] [Google Scholar]
- 14.Klein, R. S., C. A. Harris, C. B. Small, B. Moll, M. Lesser, and G. H. Friedland. 1984. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N. Engl. J. Med. 311:354-357. [DOI] [PubMed] [Google Scholar]
- 15.Knight, L., and J. Fletcher. 1971. Growth of Candida albicans in saliva: stimulation by glucose associated with antibiotics, corticosteriods and diabetes mellitus. J. Infect. Dis. 123:371-377. [DOI] [PubMed] [Google Scholar]
- 16.Laskaris, G., M. Hadjivassiliou, and J. Stratigos. 1992. Oral signs and symptoms in 160 Greek HIV-infected patients. J. Oral Pathol. 21:120-123. [DOI] [PubMed] [Google Scholar]
- 17.Leigh, J. E., M. Barousse, R. K. Swoboda, T. Myers, S. Hager, N. A. Wolf, J. L. Cutright, J. Thompson, Sobel, J. D., and P. L. Fidel, Jr. 2001. Candida-specific systemic cell-mediated immune reactivities in HIV-infected persons with or without mucosal candidiaisis. J. Infect. Dis. 183:277-285. [DOI] [PubMed] [Google Scholar]
- 18.Leigh, J. E., C. Steele, F. L. Wormley, Jr., W. Luo, R. A. Clark, W. R. Gallaher, and P. L. Fidel, Jr. 1998. Th1/Th2 cytokine expression in saliva of HIV-positive and HIV-negative individuals: a pilot study in HIV-positive individuals with oropharyngeal candidiasis. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19:373-380. [DOI] [PubMed] [Google Scholar]
- 19.Levy, J. A., F. Hsueh, D. J. Blackbourn, D. Wara, and P. S. Weintrub. 1998. CD8 cell noncytotoxic antiviral activity in human immunodeficiency virus-infected and -uninfected children. J. Infect. Dis. 177:470-472. [DOI] [PubMed] [Google Scholar]
- 20.Macher, A. M. 1988. The pathology of AIDS. Public Health Rep. 103:246-254. [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai, Y., N. Takeshita, and T. Saku. 1992. Histopathologic and ultrastructural studies of oral mucosa with Candida infection. J. Oral Pathol. Med. 21:171-175. [DOI] [PubMed] [Google Scholar]
- 22.Odds, F. C. 1988. Chronic mucocutaneous candidiosis. In Candida and candidosis, p. 104-110. University Park Press, Baltimore, Md.
- 23.Paterson, P. Y., R. Semo, G. Blumenschein, and J. Swelstad. 1971. Mucocutaneous candidiasis, anergy and a plasma inhibitor of cellular immunity: reversal after amphotericin B therapy. Clin. Exp. Immunol. 9:595-602. [PMC free article] [PubMed] [Google Scholar]
- 24.Patton, L. L., R. G. McKaig, R. P. Strauss, and J. J. Enron. 1998. Oral manifestations of HIV in a southeast USA population. Oral Dis. 4:164-169. [DOI] [PubMed] [Google Scholar]
- 25.Reichart, P. A., L. P. Samaranayake, and H. P. Philipsen. 2000. Pathology and clinical correlates in oral candidiasis and its variants: a review. Oral Dis. 6:85-91. [DOI] [PubMed] [Google Scholar]
- 26.Revankar, S. G., S. E. Sanche, O. P. Dib, M. Caceres, and T. F. Patterson. 1998. Effect of highly active antiretroviral therapy on recurrent oropharyngeal candidiasis in HIV-infected patients. AIDS 12:2511-2513. [PubMed] [Google Scholar]
- 27.Romagnoli, P., N. Pimpinelli, M. Mori, P. A. Reichart, L. R. Eversole, and G. Ficarra. 1997. Immunocompetent cells in oral candidiasis of HIV-infected patients: an immunohistochemical and electron microscopical study. Oral Dis. 3:99-105. [DOI] [PubMed] [Google Scholar]
- 28.Romani, L., S. Mocci, C. Bietta, L. Lanfaloni, P. Puccetti, and F. Bistoni. 1991. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect. Immun. 59:4647-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slavinsky III, J., T. Myers, R. K. Swoboda, J. E. Leigh, S. Hager, and P. L. Fidel, Jr. 2002. Th1/Th2 cytokine profiles in saliva of HIV-positive smokers with oropharyngeal candidiasis. Oral Microbiol. Immunol. 17:38-43. [DOI] [PubMed] [Google Scholar]
- 30.Steele, C., J. E. Leigh, R. K. Swoboda, and P. L. Fidel, Jr. 2000. Growth inhibition of Candida by human oral epithelial cells. J. Infect. Dis. 182:1479-1485. [DOI] [PubMed] [Google Scholar]
- 31.Stranford, S. A., J. Skurnick, D. Louria, D. Osmond, S. Y. Chang, J. Sninsky, G. Ferrari, K. Weinhold, C. Lindquist, and J. A. Levy. 1999. Lack of infection in HIV-exposed individuals is associated with a strong CD8+ cell noncytotoxic anti-HIV response. Proc. Natl. Acad. Sci. USA 96:1030-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams, D. W., A. J. C. Potts, M. J. Wilson, J. B. Matthews, and M. A. O. Lewis. 1997. Characterisation of the inflammatory cell infiltrate in chronic hyperplastic candidosis of the oral mucosa. J. Oral Pathol. Med. 26:83-89. [DOI] [PubMed] [Google Scholar]
- 33.Wozniak, K. L., J. E. Leigh, S. Hager, R. K. Swoboda, and P. L. Fidel, Jr. 2002. A comprehensive study of Candida-specific antibodies in saliva of HIV-infected persons with oropharyngeal candidiasis. J. Infect. Dis. 185:1269-1276. [DOI] [PubMed] [Google Scholar]