Abstract
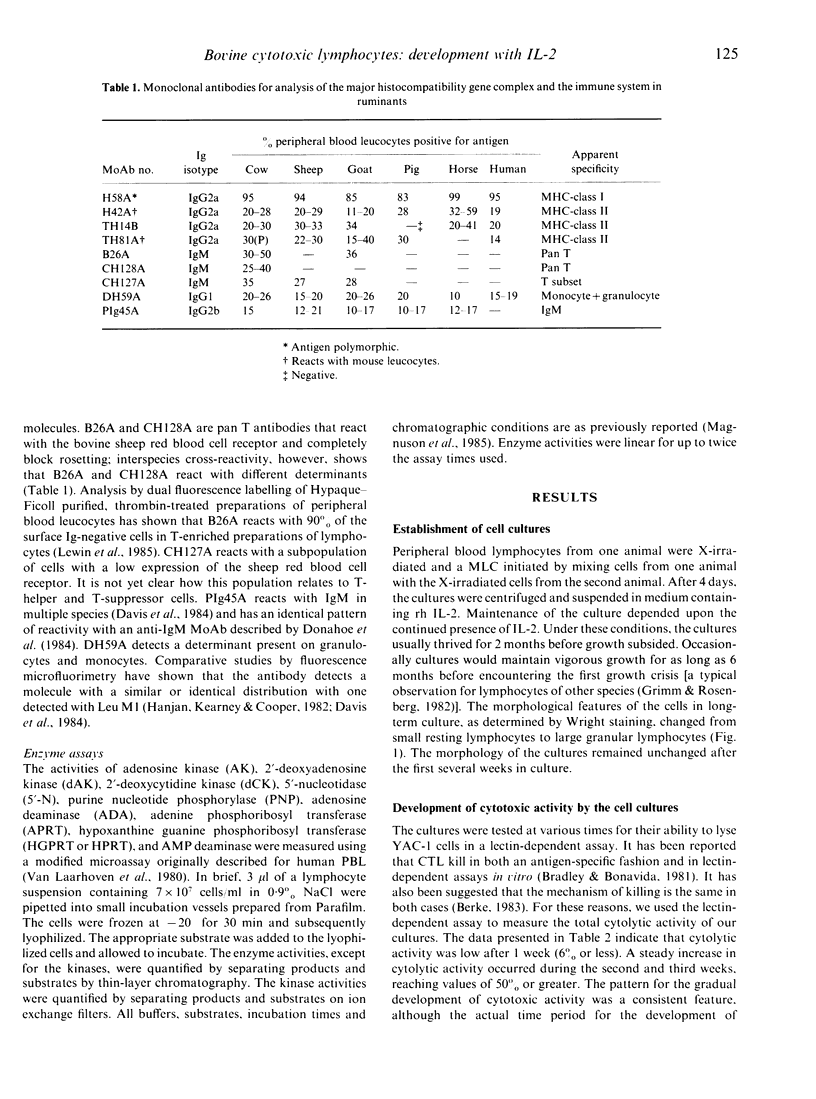
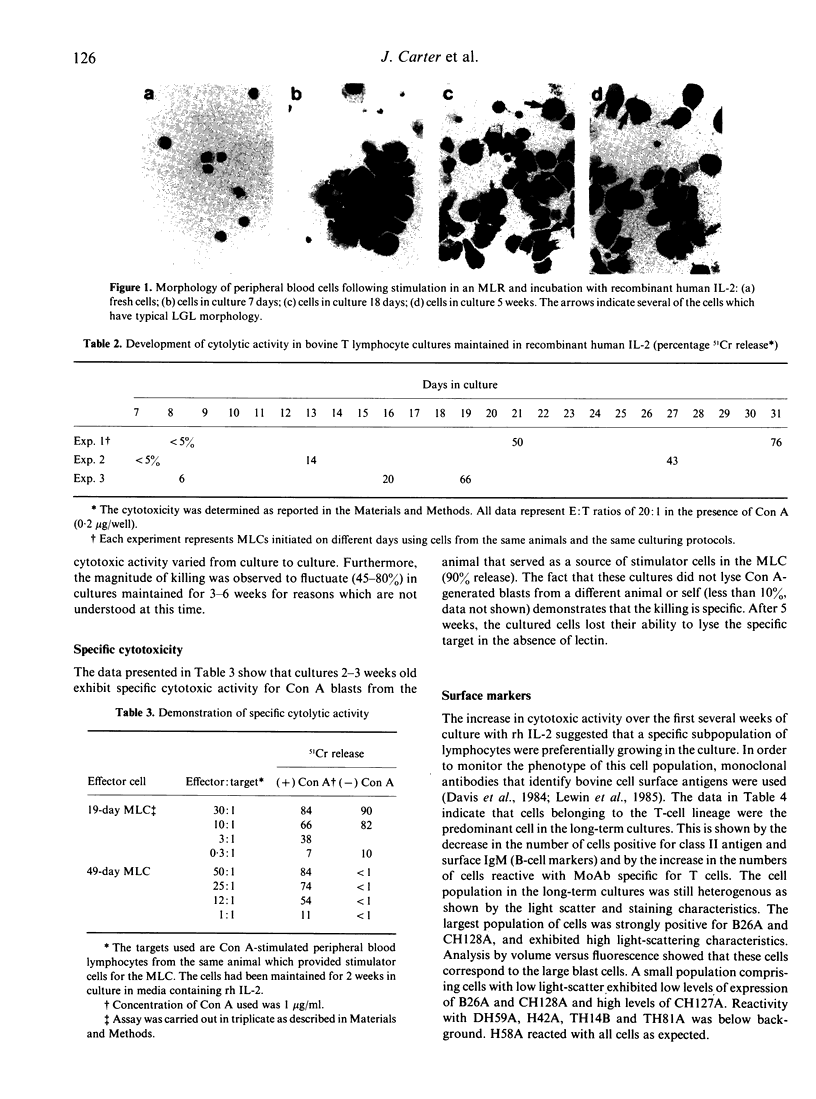
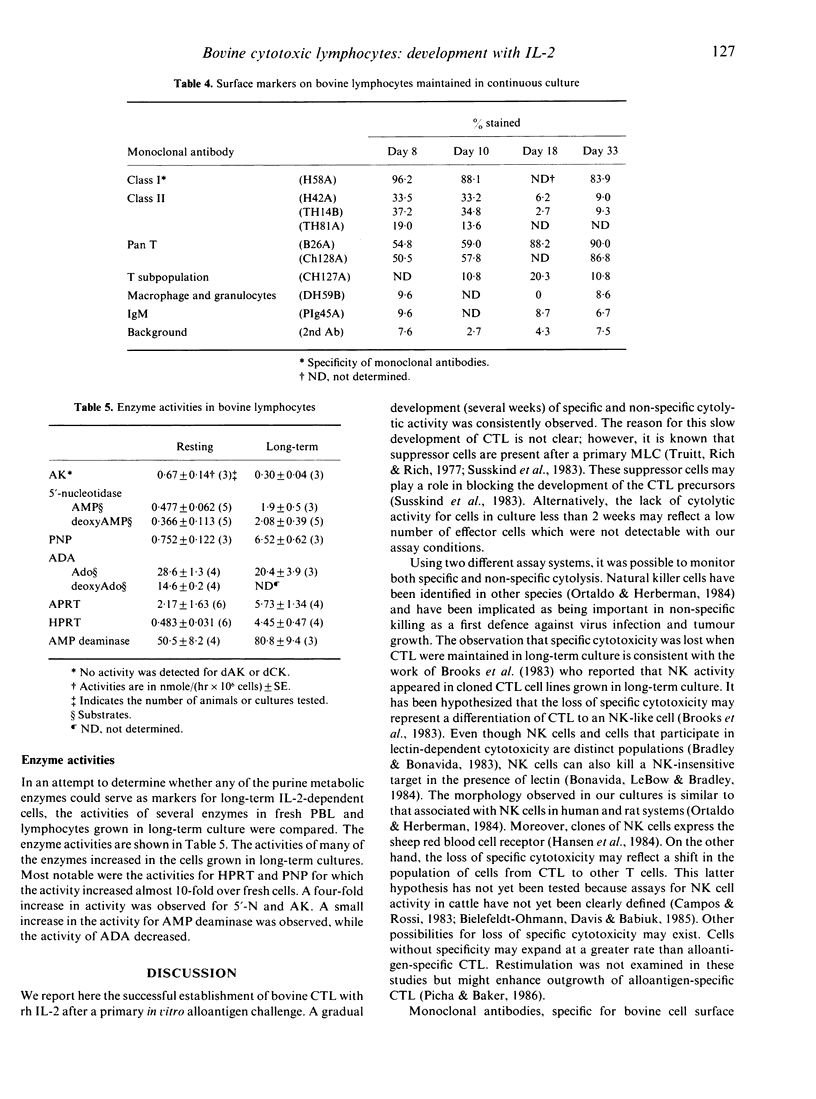
Long-term bovine lymphocyte cultures were initiated by stimulation with alloantigens and maintained in continuous culture using medium containing recombinant human interleukin-2 (rh IL-2). The development of specific and lectin-dependent killing was monitored following primary alloantigen challenge. Cytolytic activity was barely detectable after 7 days of culture, but gradually increased with peak activity occurring after 21 days of culture. A panel of monoclonal antibodies (MoAb) was used to determine whether a shift in the antigen phenotype of the cell population occurred during culture. The primary cell type that grew in culture was of the T-cell lineage with minimal or no expression of class II antigens. The activities of adenosine deaminase (ADA), purine nucleotide phosphorylase (PNP), adenosine kinase (AK), deoxyadenosine kinase (dAK), deoxycytidine kinase (dCK), 5'-nucleotidase (5'-N), AMP deaminase, hypoxanthine-guanine phosphoribosyl transferase (HGPRT or HPRT), and adenine phosphoribosyl transferase (APRT) were measured by microassay in resting peripheral blood lymphocytes (PBL) and in cells from long-term cultures. Large increases in the activities of PNP and HPRT with a decrease in the activity of ADA were observed. The data show that long-term cultures of lymphocytes can be readily generated, and that sequential changes in antigenic phenotype and function can be monitored and correlated with quantitative changes in enzyme activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton R. W., Goldschneider I. Nucleotide-metabolizing enzymes and lymphocyte differentiation. Mol Cell Biochem. 1979 Dec 14;28(1-3):135–147. doi: 10.1007/BF00223363. [DOI] [PubMed] [Google Scholar]
- Barton R., Martiniuk F., Hirschhorn R., Goldschneider I. Inverse relationship between adenosine deaminase and purine nucleoside phosphorylase in rat lymphocyte populations. Cell Immunol. 1980 Jan;49(1):208–214. doi: 10.1016/0008-8749(80)90071-4. [DOI] [PubMed] [Google Scholar]
- Berke G. Cytotoxic T-lymphocytes. How do they function? Immunol Rev. 1983;72:5–42. doi: 10.1111/j.1600-065x.1983.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Berke G., Hu V., McVey E., Clark W. R. T lymphocyte-mediated cytolysis. I. A common mechanism for target recognition in specific and lectin-dependent cytolysis. J Immunol. 1981 Aug;127(2):776–781. [PubMed] [Google Scholar]
- Bonavida B., Lebow L. T., Bradley T. P. Mechanism of cell-mediated cytotoxicity at the single cell level. VI. Direct assessment of the cytotoxic potential of human peripheral blood non-lytic effector-target cell conjugates. J Immunol. 1984 Feb;132(2):594–598. [PubMed] [Google Scholar]
- Bradley T. P., Bonavida B. Mechanism of cell-mediated cytotoxicity at the single-cell level. VII. Trigger of the lethal hit event is distinct for NK/K and LDCC effector cells as measured in the two-target conjugate assay. Cell Immunol. 1984 Jan;83(1):199–207. doi: 10.1016/0008-8749(84)90239-9. [DOI] [PubMed] [Google Scholar]
- Brooks C. G., Urdal D. L., Henney C. S. Lymphokine-driven "differentiation" of cytotoxic T-cell clones into cells with NK-like specificity: correlations with display of membrane macromolecules. Immunol Rev. 1983;72:43–72. doi: 10.1111/j.1600-065x.1983.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Campos M., Rossi C. R. Cell-mediated cytotoxicity of bovine mononuclear cells to IBRV-infected cells: dependence on Sephadex G-10 adherent cells. Vet Immunol Immunopathol. 1985 Apr;8(4):363–375. doi: 10.1016/0165-2427(85)90006-6. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Wasson D. B. Differences in deoxyadenosine metabolism in human and mouse lymphocytes. J Immunol. 1980 Jan;124(1):8–12. [PubMed] [Google Scholar]
- Engleman E. G., Warnke R., Fox R. I., Dilley J., Benike C. J., Levy R. Studies of a human T lymphocyte antigen recognized by a monoclonal antibody. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1791–1795. doi: 10.1073/pnas.78.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Hanjan S. N., Kearney J. F., Cooper M. D. A monoclonal antibody (MMA) that identifies a differentiation antigen on human myelomonocytic cells. Clin Immunol Immunopathol. 1982 May;23(2):172–188. doi: 10.1016/0090-1229(82)90106-4. [DOI] [PubMed] [Google Scholar]
- Ma D. D., Sylwestrowicz T. A., Granger S., Massaia M., Franks R., Janossy G., Hoffbrand A. V. Distribution of terminal deoxynucleotidyl transferase and purine degradative and synthetic enzymes in subpopulations of human thymocytes. J Immunol. 1982 Oct;129(4):1430–1435. [PubMed] [Google Scholar]
- Magnuson N. S., Perryman L. E., Mason P. H., Marta K. M. Distribution of enzymes of purine metabolism in lymphocytes of horse, Equus caballus. Comp Biochem Physiol B. 1985;81(2):459–465. doi: 10.1016/0305-0491(85)90342-6. [DOI] [PubMed] [Google Scholar]
- Minkowski M. D., Castellazzi M., Buttin G. Lack of adenosine deaminase activity in cultured murine cytotoxic T lymphocytes. J Immunol. 1984 Jul;133(1):52–58. [PubMed] [Google Scholar]
- Ortaldo J. R., Herberman R. B. Heterogeneity of natural killer cells. Annu Rev Immunol. 1984;2:359–394. doi: 10.1146/annurev.iy.02.040184.002043. [DOI] [PubMed] [Google Scholar]
- Peters G. J., Veerkamp J. H. Purine and pyrimidine metabolism in peripheral blood lymphocytes. Int J Biochem. 1983;15(2):115–123. doi: 10.1016/0020-711x(83)90051-4. [DOI] [PubMed] [Google Scholar]
- Ratner L., Gallo R. C., Wong-Staal F. HTLV-III, LAV, ARV are variants of same AIDS virus. Nature. 1985 Feb 21;313(6004):636–637. doi: 10.1038/313636c0. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Broder S., Essex M., Gallo R. C. Human T-cell leukemia virus: its discovery and role in leukemogenesis and immunosuppression. Adv Intern Med. 1984;30:1–27. [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Susskind B. M., Merluzzi V. J., Faanes R. B., Palladino M. A., Choi Y. S. Regulatory mechanisms in cytotoxic T lymphocyte development. I. A suppressor T cell subset that regulates the proliferative stage of CTL development. J Immunol. 1983 Feb;130(2):527–532. [PubMed] [Google Scholar]
- Thompson L. F., Saxon A., O'Connor R. D., Fox R. I. Ecto-5'-nucleotidase activity in human T cell subsets. Decreased numbers of ecto-5'-nucleotidase positive cells from both OKT4+ and OKT8+ cells in patients with hypogammaglobulinemia. J Clin Invest. 1983 Apr;71(4):892–899. doi: 10.1172/JCI110843. [DOI] [PMC free article] [PubMed] [Google Scholar]