Abstract
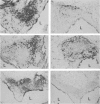
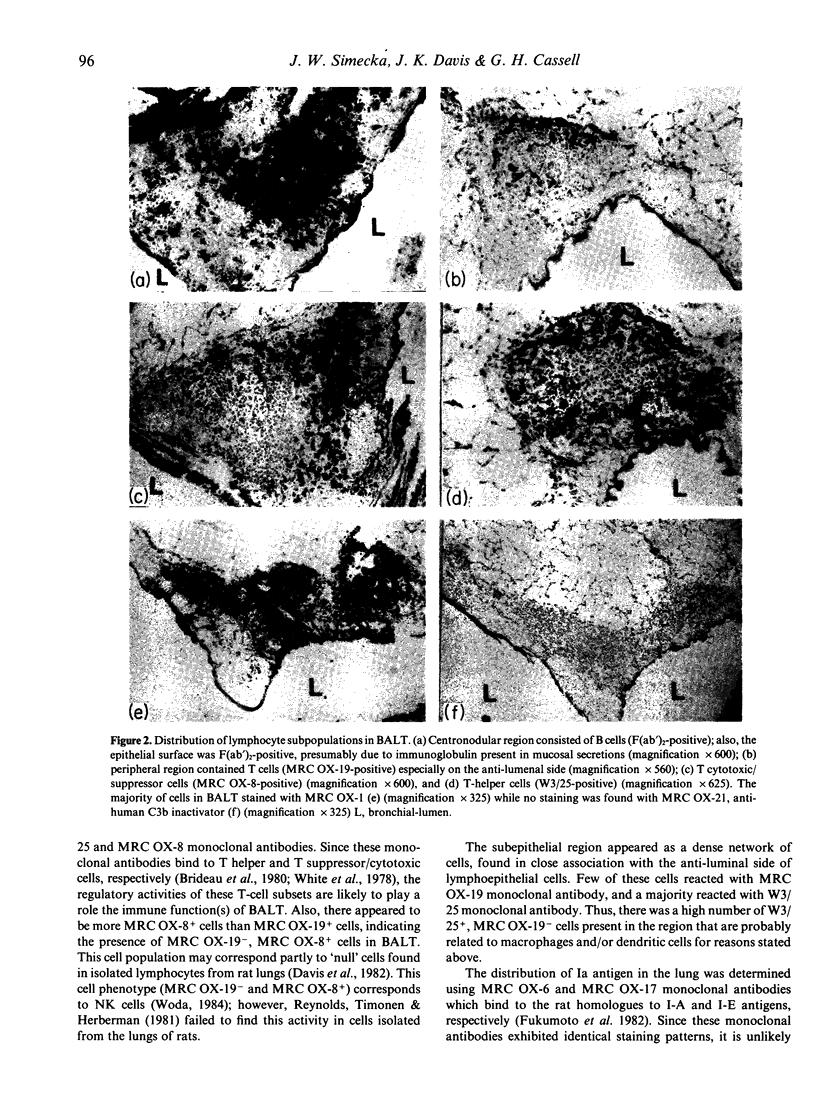
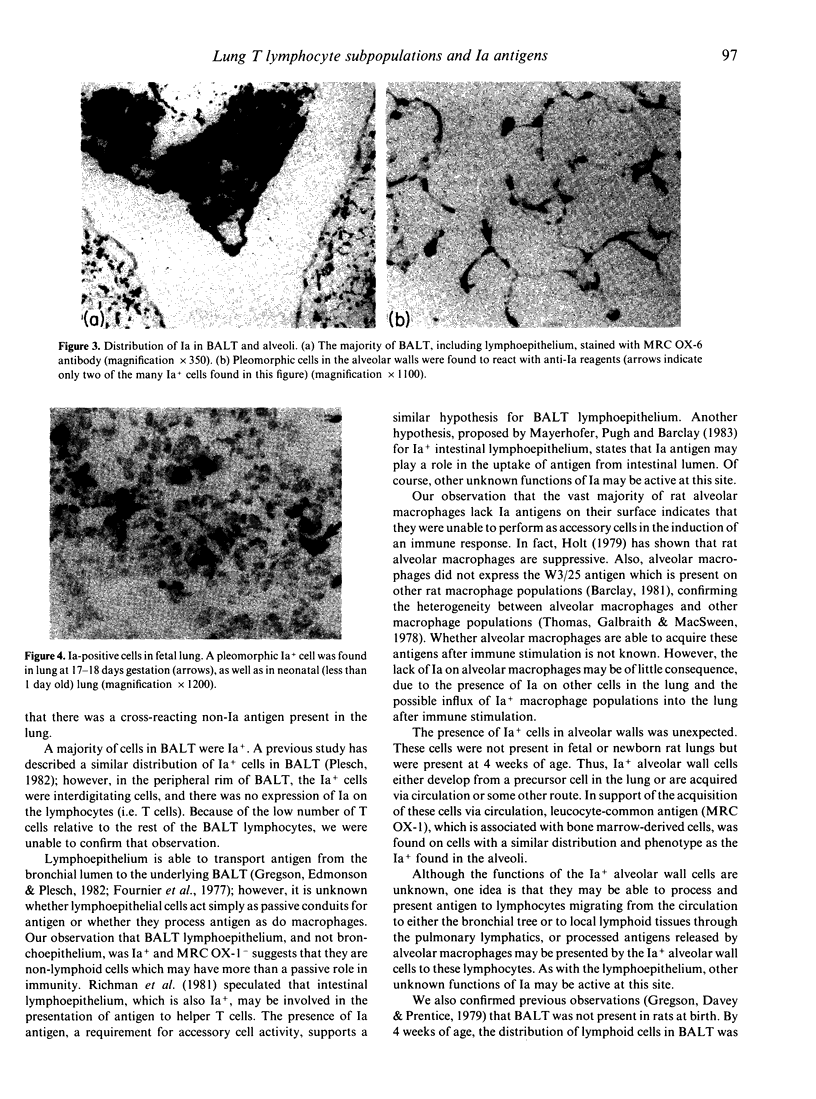
In order to examine the mechanism of specific immunity in the lung, the distribution of Ia antigens and T lymphocyte populations was determined using immunoperoxidase-staining of cryostat sections of lungs from specific pathogen-free rats. BALT was found to be divided into three regions of lymphoid tissue. The central region was primarily composed of B cells, and was surrounded by a peripheral region of T cells (MRC OX-19+) which included both T helper (W3/25+) and T suppressor/cytotoxic (MRC OX-8+) cells. The subepithelial region contained a dense network of W3/25+, non-T cells. A majority of BALT cells, including the lymphoepithelial cells, were Ia+. The alveolar walls were found to contain numerous Ia+ dendritic-shaped cells. Alveolar macrophages found in sections, as well as those collected using bronchoalveolar lavage, were Ia- and W3/25-. Mechanisms for the induction of immunity within both BALT and the alveolar region are proposed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N. The localization of populations of lymphocytes defined by monoclonal antibodies in rat lymphoid tissues. Immunology. 1981 Apr;42(4):593–600. [PMC free article] [PubMed] [Google Scholar]
- Beacham C. H., Daniele R. P. Migration of recently divided B and T lymphocytes to peritoneum and lung. Cell Immunol. 1982 Dec;74(2):284–293. doi: 10.1016/0008-8749(82)90029-6. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Cassell G. H., Lindsey J. R., Davis J. K., Davidson M. K., Brown M. B., Mayo J. G. Detection of natural Mycoplasma pulmonis infection in rats and mice by an enzyme linked immunosorbent assay (ELISA). Lab Anim Sci. 1981 Dec;31(6):676–682. [PubMed] [Google Scholar]
- Clancy R., Bienenstock J. The proliferative response of bronchus-associated lymphoid tissue after local and systemic immunization. J Immunol. 1974 Jun;112(6):1997–2001. [PubMed] [Google Scholar]
- Dallman M. J., Thomas M. L., Green J. R. MRC OX-19: a monoclonal antibody that labels rat T lymphocytes and augments in vitro proliferative responses. Eur J Immunol. 1984 Mar;14(3):260–267. doi: 10.1002/eji.1830140311. [DOI] [PubMed] [Google Scholar]
- Davis J. K., Cassell G. H. Murine respiratory mycoplasmosis in LEW and F344 rats: strain differences in lesion severity. Vet Pathol. 1982 May;19(3):280–293. doi: 10.1177/030098588201900306. [DOI] [PubMed] [Google Scholar]
- Davis J. K., Delozier K. M., Asa D. K., Minion F. C., Cassell G. H. Interactions between murine alveolar macrophages and Mycoplasma pulmonis in vitro. Infect Immun. 1980 Aug;29(2):590–599. doi: 10.1128/iai.29.2.590-599.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Thorp R. B., Maddox P. A., Brown M. B., Cassell G. H. Murine respiratory mycoplasmosis in F344 and LEW rats: evolution of lesions and lung lymphoid cell populations. Infect Immun. 1982 May;36(2):720–729. doi: 10.1128/iai.36.2.720-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto T., McMaster W. R., Williams A. F. Mouse monoclonal antibodies against rat major histocompatibility antigens. Two Ia antigens and expression of Ia and class I antigens in rat thymus. Eur J Immunol. 1982 Mar;12(3):237–243. doi: 10.1002/eji.1830120313. [DOI] [PubMed] [Google Scholar]
- Gregson R. L., Davey M. J., Prentice D. E. Bronchus-associated lymphoid tissue (BALT) in the laboratory-bred and wild rat, Rattus norvegicus. Lab Anim. 1979 Jul;13(3):239–243. doi: 10.1258/002367779780937735. [DOI] [PubMed] [Google Scholar]
- Gregson R. L., Edmondson N. A., Plesch B. Preferential uptake of soluble antigen by respiratory tract epithelium overlying bronchus-associated lymphoid tissue in the rat. Adv Exp Med Biol. 1982;149:499–505. doi: 10.1007/978-1-4684-9066-4_70. [DOI] [PubMed] [Google Scholar]
- Holt P. G. Alveolar macrophages. III. Studies on the mechanisms of inhibition of T-cell proliferation. Immunology. 1979 Jun;37(2):437–445. [PMC free article] [PubMed] [Google Scholar]
- Hsiung L., Barclay A. N., Brandon M. R., Sim E., Porter R. R. Purification of human C3b inactivator by monoclonal-antibody affinity chromatography. Biochem J. 1982 Apr 1;203(1):293–298. doi: 10.1042/bj2030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensenius J. C., Williams A. F. The binding of anti-immunoglobulin antibodies to rat thymocytes and thoracic duct lymphocytes. Eur J Immunol. 1974 Feb;4(2):91–97. doi: 10.1002/eji.1830040207. [DOI] [PubMed] [Google Scholar]
- Lindsey J. R., Baker H. J., Overcash R. G., Cassell G. H., Hunt C. E. Murine chronic respiratory disease. Significance as a research complication and experimental production with Mycoplasma pulmonis. Am J Pathol. 1971 Sep;64(3):675–708. [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G., Pugh C. W., Barclay A. N. The distribution, ontogeny and origin in the rat of Ia-positive cells with dendritic morphology and of Ia antigen in epithelia, with special reference to the intestine. Eur J Immunol. 1983 Feb;13(2):112–122. doi: 10.1002/eji.1830130206. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Plesch B. E. Histology and immunohistochemistry of bronchus associated lymphoid tissue (BALT) in the rat. Adv Exp Med Biol. 1982;149:491–497. doi: 10.1007/978-1-4684-9066-4_69. [DOI] [PubMed] [Google Scholar]
- Reynolds C. W., Timonen T., Herberman R. B. Natural killer (NK) cell activity in the rat. I. Isolation and characterization of the effector cells. J Immunol. 1981 Jul;127(1):282–287. [PubMed] [Google Scholar]
- Richman L. K., Graeff A. S., Yarchoan R., Strober W. Simultaneous induction of antigen-specific IgA helper T cells and IgG suppressor T cells in the murine Peyer's patch after protein feeding. J Immunol. 1981 Jun;126(6):2079–2083. [PubMed] [Google Scholar]
- Rácz P., Tenner-Rácz K., Myrvik Q. N., Fainter L. K. Functional architecture of bronchial associated lymphoid tissue and lymphoepithelium in pulmonary cell-mediated reactions in the rabbit. J Reticuloendothel Soc. 1977 Jul;22(1):59–83. [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Thomas M. A., Galbraith I., MacSween R. N. Heterogeneity of rat peritoneal and alveolar macrophage populations: spontaneous rosette formation using sheep and chicken red blood cells. J Reticuloendothel Soc. 1978 Jan;23(1):43–52. [PubMed] [Google Scholar]
- White R. A., Mason D. W., Williams A. F., Galfre G., Milstein C. T-lymphocyte heterogeneity in the rat: separation of functional subpopulations using a monoclonal antibody. J Exp Med. 1978 Sep 1;148(3):664–673. doi: 10.1084/jem.148.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Woda B. A., McFadden M. L., Welsh R. M., Bain K. M. Separation and isolation of rat natural killer (NK) cells from T cells with monoclonal antibodies. J Immunol. 1984 May;132(5):2183–2184. [PubMed] [Google Scholar]