Abstract
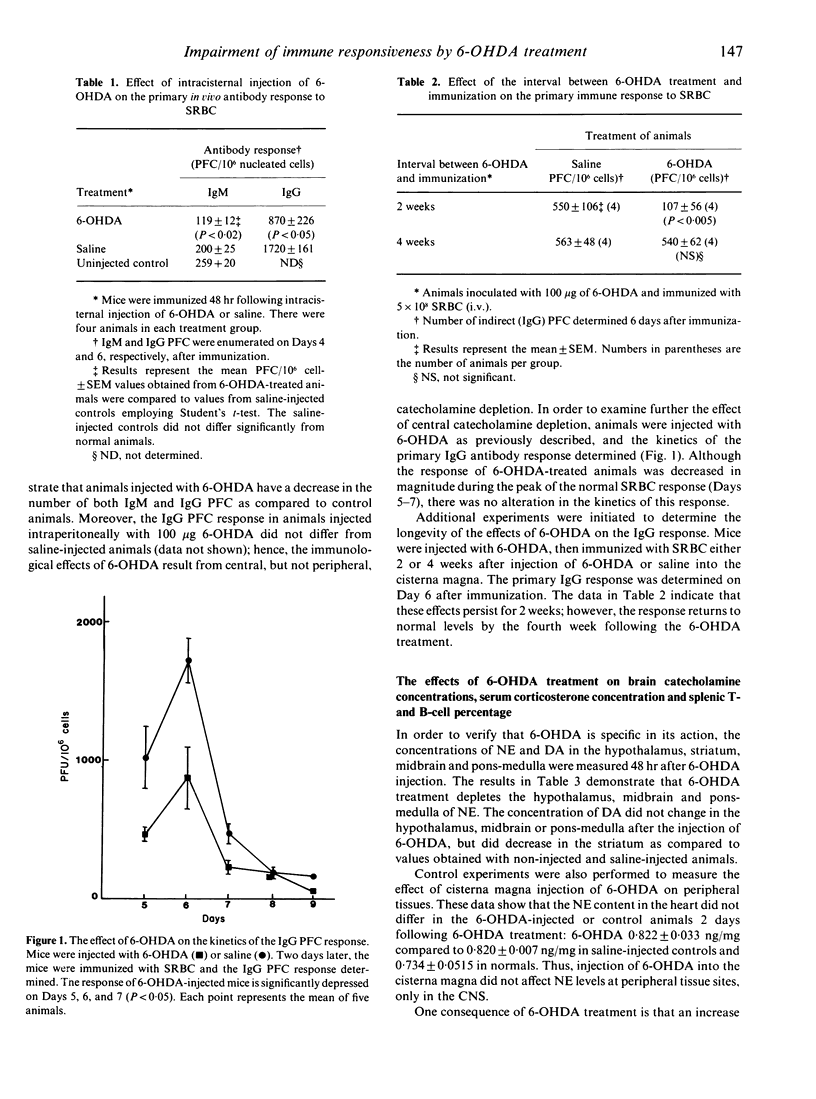
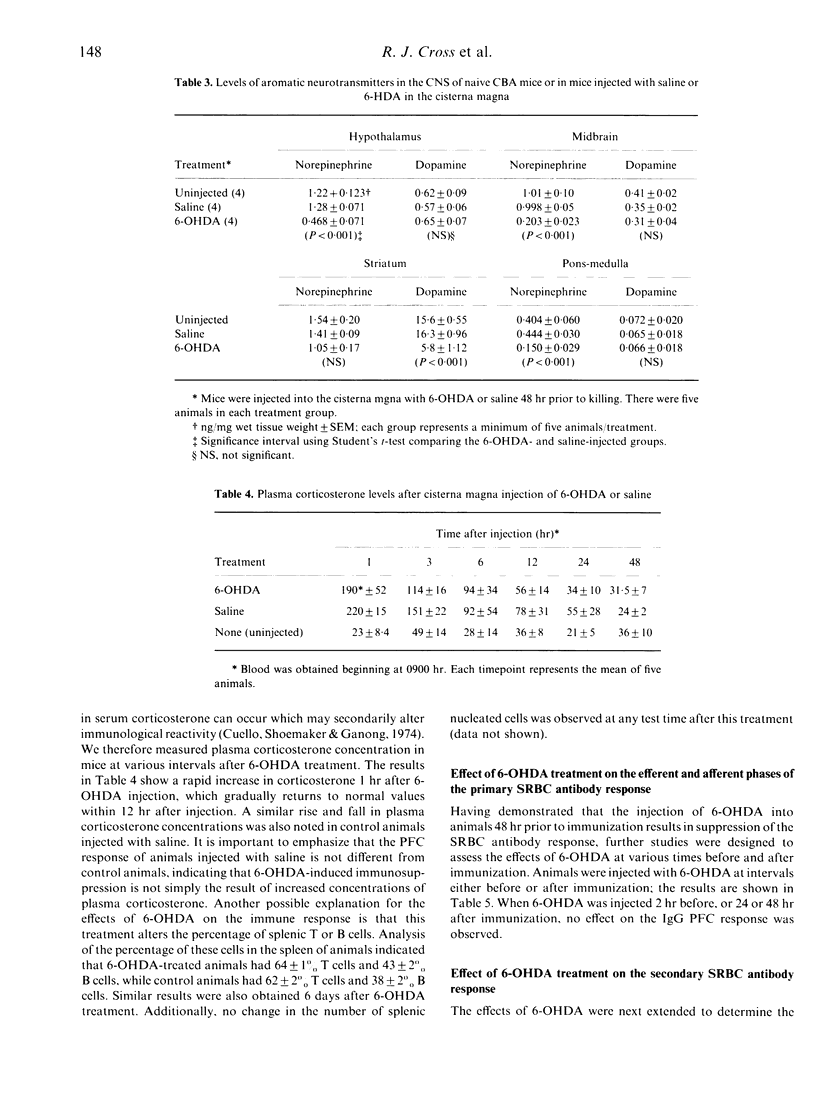
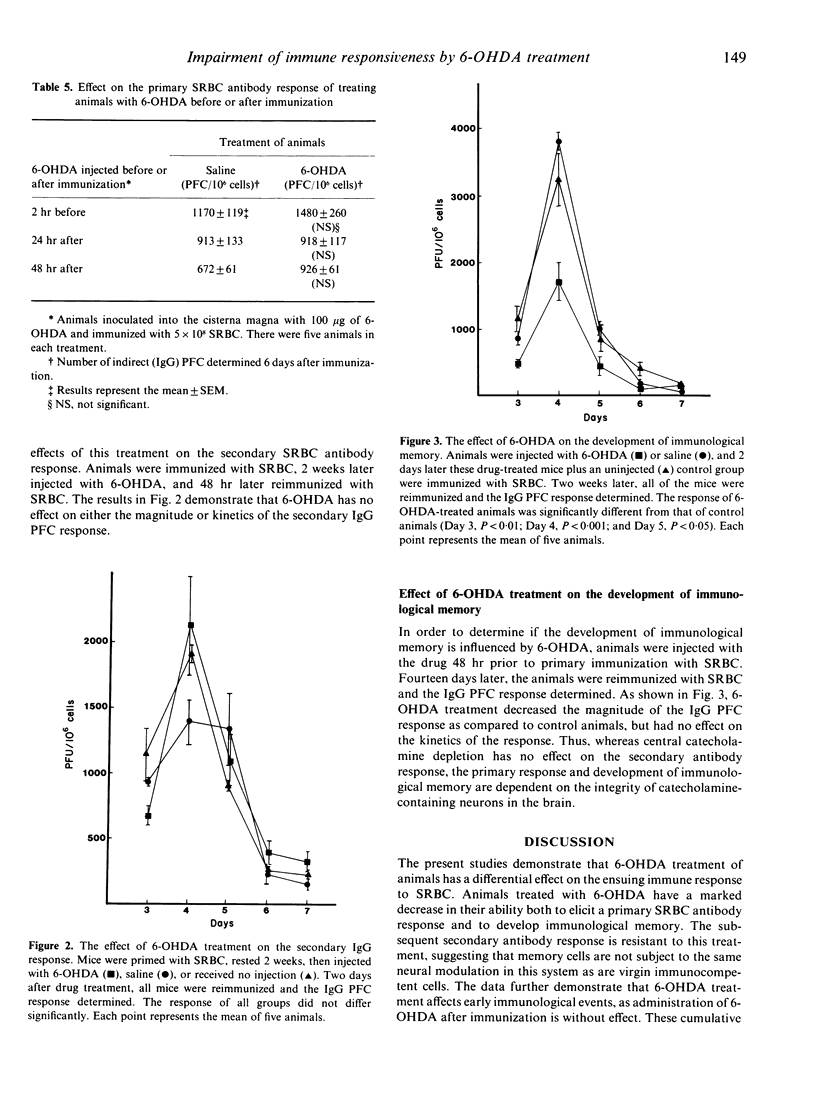
Previous studies from this laboratory and others show that perturbations of the central nervous system modulate immune function. In addition, reports from several investigators indicate that depletion of the neurotransmitter norepinephrine (NE) in peripheral nerves by injecting the neurotoxin 6-hydroxydopamine (6-OHDA), can enhance or suppress the antibody response. However, immunocompetence following brain depletion of catecholamines has not been investigated. In this study, we investigated the effects of injecting 6-OHDA into the cisterna magna of male CBA/J mice, and determined the effects of this treatment on both the IgM and IgG antibody responses to sheep red blood cells (SRBC). Both responses are suppressed compared to saline-injected control or normal animals. Animals treated with 6-OHDA have decreased levels of NE in the midbrain, pons-medulla and hypothalamus, while dopamine levels did not change in these brain regions but was decreased in the striatum. The percentage of splenic T cells and B cells was not affected by 6-OHDA treatment. Although there is a marked increase in plasma corticosterone levels in 6-OHDA-treated mice, saline-injected control animals have equivalent increases in plasma corticosterone without concomitant impairment of the immune response. Thus, the decline in immune responsiveness following 6-OHDA treatment does not result from corticosterone-induced immunosuppression. Analysis of the kinetics of the primary IgG response following 6-OHDA treatment indicates that the magnitude, but not the kinetics, of the response decreases. Experiments to determine the effects of 6-OHDA on the afferent and efferent phrases of the response demonstrate that it is effective only when administered prior to immunization, and thus must inhibit early events involved in the initiation of the response. Additional experiments show that mice can be immunized 2 weeks following brain catecholamines depletion and still exhibit a decreased antibody response. However, the response returns to normal levels if immunization is delayed 4 weeks after injection. Further experiments demonstrated that 6-OHDA treatment has no effect on the secondary antibody response, but does inhibit the development of immunological memory. Collectively, these results indicate that 6-OHDA treatment has a profound inhibitory effect on the induction of the primary antibody response and immunological memory development, but is without effect on the secondary antibody response. The data further substantiate the existence of a link between the brain and the immune response.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrenbrecht S. Specific binding of growth hormone to thymocytes. Nature. 1974 Nov 15;252(5480):255–257. doi: 10.1038/252255a0. [DOI] [PubMed] [Google Scholar]
- Bell L. J., Iversen L. L., Uretsky N. J. Time course of the effects of 6-hydroxydopamine on catecholamine-containing neurones in rat hypothalamus and striatum. Br J Pharmacol. 1970 Dec;40(4):790–799. doi: 10.1111/j.1476-5381.1970.tb10655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky H. O., del Rey A., Sorkin E., Da Prada M., Keller H. H. Immunoregulation mediated by the sympathetic nervous system. Cell Immunol. 1979 Dec;48(2):346–355. doi: 10.1016/0008-8749(79)90129-1. [DOI] [PubMed] [Google Scholar]
- Brooks W. H., Cross R. J., Roszman T. L., Markesbery W. R. Neuroimmunomodulation: neural anatomical basis for impairment and facilitation. Ann Neurol. 1982 Jul;12(1):56–61. doi: 10.1002/ana.410120111. [DOI] [PubMed] [Google Scholar]
- Charnay Y., Léger L., Dray F., Bérod A., Jouvet M., Pujol J. F., Dubois P. M. Evidence for the presence of enkephalin in catecholaminergic neurones of cat locus coeruleus. Neurosci Lett. 1982 May 28;30(2):147–151. doi: 10.1016/0304-3940(82)90287-7. [DOI] [PubMed] [Google Scholar]
- Cross R. J., Markesbery W. R., Brooks W. H., Roszman T. L. Hypothalamic-immune interactions. I. The acute effect of anterior hypothalamic lesions on the immune response. Brain Res. 1980 Aug 25;196(1):79–87. doi: 10.1016/0006-8993(80)90717-9. [DOI] [PubMed] [Google Scholar]
- Cross R. J., Markesbery W. R., Brooks W. H., Roszman T. L. Hypothalamic-immune interactions: neuromodulation of natural killer activity by lesioning of the anterior hypothalamus. Immunology. 1984 Feb;51(2):399–405. [PMC free article] [PubMed] [Google Scholar]
- Cuello A. C., Shoemaker W. J., Ganong W. F. Effect of 6-hydroxydopamine on hypothalamic norepinephrine and dopamine content, ultrastructure of the median eminence, and plasma corticosterone. Brain Res. 1974 Sep 20;78(1):57–69. doi: 10.1016/0006-8993(74)90353-9. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Everitt B. J., Hökfelt T., Terenius L., Tatemoto K., Mutt V., Goldstein M. Differential co-existence of neuropeptide Y (NPY)-like immunoreactivity with catecholamines in the central nervous system of the rat. Neuroscience. 1984 Feb;11(2):443–462. doi: 10.1016/0306-4522(84)90036-8. [DOI] [PubMed] [Google Scholar]
- Faith R. E., Liang H. J., Murgo A. J., Plotnikoff N. P. Neuroimmunomodulation with enkephalins: enhancement of human natural killer (NK) cell activity in vitro. Clin Immunol Immunopathol. 1984 Jun;31(3):412–418. doi: 10.1016/0090-1229(84)90093-x. [DOI] [PubMed] [Google Scholar]
- Fenske M., Wuttke W. Effects of intraventricular 6-hydroxydopamine injections on serum prolactin and LH levels: absence of stress-induced pituitary prolactin release. Brain Res. 1976 Mar 5;104(1):63–70. doi: 10.1016/0006-8993(76)90647-8. [DOI] [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966 Aug;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Gupta S., Fikrig S. M., Noval M. S. Immunological studies in patients with isolated growth hormone deficiency. Clin Exp Immunol. 1983 Oct;54(1):87–90. [PMC free article] [PubMed] [Google Scholar]
- Hall N. R., McClure J. E., Hu S. K., Tare N. S., Seals C. M., Goldstein A. L. Effects of 6-hydroxydopamine upon primary and secondary thymus dependent immune responses. Immunopharmacology. 1982 Oct;5(1):39–48. doi: 10.1016/0162-3109(82)90035-2. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Flier J., Itin A., Kahn C. R., Roth J. Radioimmunoassay of the insulin receptor: a new probe of receptor structure and function. Science. 1979 Feb 9;203(4380):544–547. doi: 10.1126/science.83675. [DOI] [PubMed] [Google Scholar]
- Hazum E., Chang K. J., Cuatrecasas P. Specific nonopiate receptors for beta-endorphin. Science. 1979 Sep 7;205(4410):1033–1035. doi: 10.1126/science.224457. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Heininger K., Grosse-Wilde H., Kalies I. Autoimmune human T lymphocytes specific for acetylcholine receptor. Nature. 1984 Jul 19;310(5974):244–246. doi: 10.1038/310244a0. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Everitt B. J., Theodorsson-Norheim E., Goldstein M. Occurrence of neurotensinlike immunoreactivity in subpopulations of hypothalamic, mesencephalic, and medullary catecholamine neurons. J Comp Neurol. 1984 Feb 1;222(4):543–559. doi: 10.1002/cne.902220407. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Lundberg J. M., Lagercrantz H., Tatemoto K., Mutt V., Lindberg J., Terenius L., Everitt B. J., Fuxe K., Agnati L. Occurrence of neuropeptide Y (NPY)-like immunoreactivity in catecholamine neurons in the human medulla oblongata. Neurosci Lett. 1983 Apr 29;36(3):217–222. doi: 10.1016/0304-3940(83)90003-4. [DOI] [PubMed] [Google Scholar]
- Jackson J. C., Cross R. J., Walker R. F., Markesbery W. R., Brooks W. H., Roszman T. L. Influence of serotonin on the immune response. Immunology. 1985 Mar;54(3):505–512. [PMC free article] [PubMed] [Google Scholar]
- Janković B. D., Isaković K. Neuro-endocrine correlates of immune response. I. Effects of brain lesions on antibody production, Arthus reactivity and delayed hypersensitivity in the rat. Int Arch Allergy Appl Immunol. 1973;45(3):360–372. [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kasahara K., Tanaka S., Ito T., Hamashima Y. Suppression of the primary immune response by chemical sympathectomy. Res Commun Chem Pathol Pharmacol. 1977 Apr;16(4):687–694. [PubMed] [Google Scholar]
- Kostrzewa R. M., Jacobowitz D. M. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974 Sep;26(3):199–288. [PubMed] [Google Scholar]
- Lopker A., Abood L. G., Hoss W., Lionetti F. J. Stereoselective muscarinic acetylcholine and opiate receptiors in human phagocytic leukocytes. Biochem Pharmacol. 1980 May 15;29(10):1361–1365. doi: 10.1016/0006-2952(80)90431-1. [DOI] [PubMed] [Google Scholar]
- Macris N. T., Schiavi R. C., Camerino M. S., Stein M. Effect of hypothalamic lesions on immune processes in the guinea pig. Am J Physiol. 1970 Nov;219(5):1205–1209. doi: 10.1152/ajplegacy.1970.219.5.1205. [DOI] [PubMed] [Google Scholar]
- Mathews P. M., Froelich C. J., Sibbitt W. L., Jr, Bankhurst A. D. Enhancement of natural cytotoxicity by beta-endorphin. J Immunol. 1983 Apr;130(4):1658–1662. [PubMed] [Google Scholar]
- Miles K., Atweh S., Otten G., Arnason B. G., Chelmicka-Schorr E. Beta-adrenergic receptors on splenic lymphocytes from axotomized mice. Int J Immunopharmacol. 1984;6(3):171–177. doi: 10.1016/0192-0561(84)90014-6. [DOI] [PubMed] [Google Scholar]
- Miles K., Quintáns J., Chelmicka-Schorr E., Arnason B. G. The sympathetic nervous system modulates antibody response to thymus-independent antigens. J Neuroimmunol. 1981 Mar;1(1):101–105. doi: 10.1016/0165-5728(81)90012-6. [DOI] [PubMed] [Google Scholar]
- O'Hearn M., Stites D. P. Inhibition of murine suppressor cell function by progesterone. Cell Immunol. 1983 Mar;76(2):340–350. doi: 10.1016/0008-8749(83)90377-5. [DOI] [PubMed] [Google Scholar]
- Payan D. G., Levine J. D., Goetzl E. J. Modulation of immunity and hypersensitivity by sensory neuropeptides. J Immunol. 1984 Apr;132(4):1601–1604. [PubMed] [Google Scholar]
- Pierpaoli W., Maestroni G. J. Pharmacological control of the hormonally modulated immune response. II. Blockade of antibody production by a combination of drugs acting on neuroendocrine functions. Its prevention by gonadotropins and corticotrophin. Immunology. 1978 Mar;34(3):419–430. [PMC free article] [PubMed] [Google Scholar]
- Russell D. H., Kibler R., Matrisian L., Larson D. F., Poulos B., Magun B. E. Prolactin receptors on human T and B lymphocytes: antagonism of prolactin binding by cyclosporine. J Immunol. 1985 May;134(5):3027–3031. [PubMed] [Google Scholar]
- Shavit Y., Lewis J. W., Terman G. W., Gale R. P., Liebeskind J. C. Opioid peptides mediate the suppressive effect of stress on natural killer cell cytotoxicity. Science. 1984 Jan 13;223(4632):188–190. doi: 10.1126/science.6691146. [DOI] [PubMed] [Google Scholar]
- Solomon G. F. Emotions, stress, the central nervous system, and immunity. Ann N Y Acad Sci. 1969 Oct 14;164(2):335–343. doi: 10.1111/j.1749-6632.1969.tb14048.x. [DOI] [PubMed] [Google Scholar]
- Sparks D. L., Slevin J. T. Determination of tyrosine, tryptophan and their metabolic derivatives by liquid chromatography-electrochemical detection: application to post mortem samples from patients with Parkinson's and Alzheimer's disease. Life Sci. 1985 Feb 4;36(5):449–457. doi: 10.1016/0024-3205(85)90257-7. [DOI] [PubMed] [Google Scholar]
- Wang Y., Joncourt F., Kristensen F., de Weck A. L. Cell cycle-related changes in number of T-lymphocyte receptors for glucocorticoids and insulin. Int J Immunopharmacol. 1984;6(2):105–110. doi: 10.1016/0192-0561(84)90004-3. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Lefkowitz R. J. Identification of beta-adrenergic receptors in human lymphocytes by (-) (3H) alprenolol binding. J Clin Invest. 1976 Jan;57(1):149–155. doi: 10.1172/JCI108254. [DOI] [PMC free article] [PubMed] [Google Scholar]


