Abstract
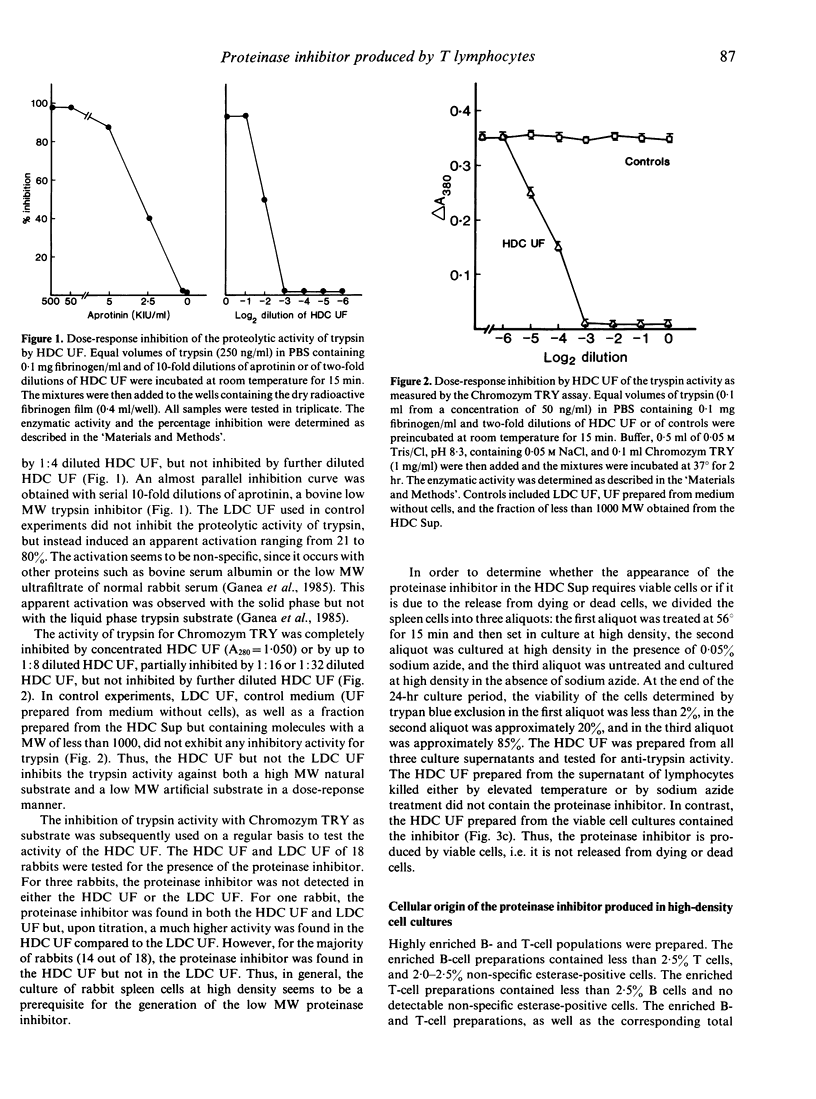
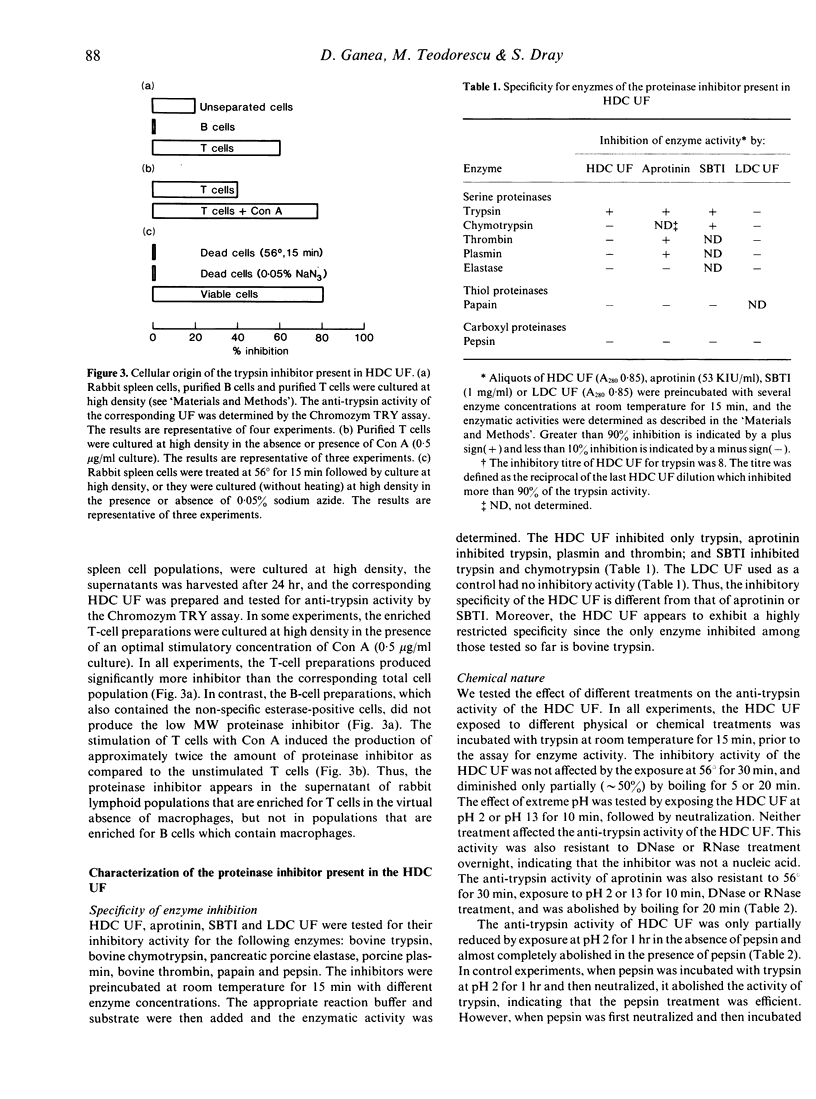
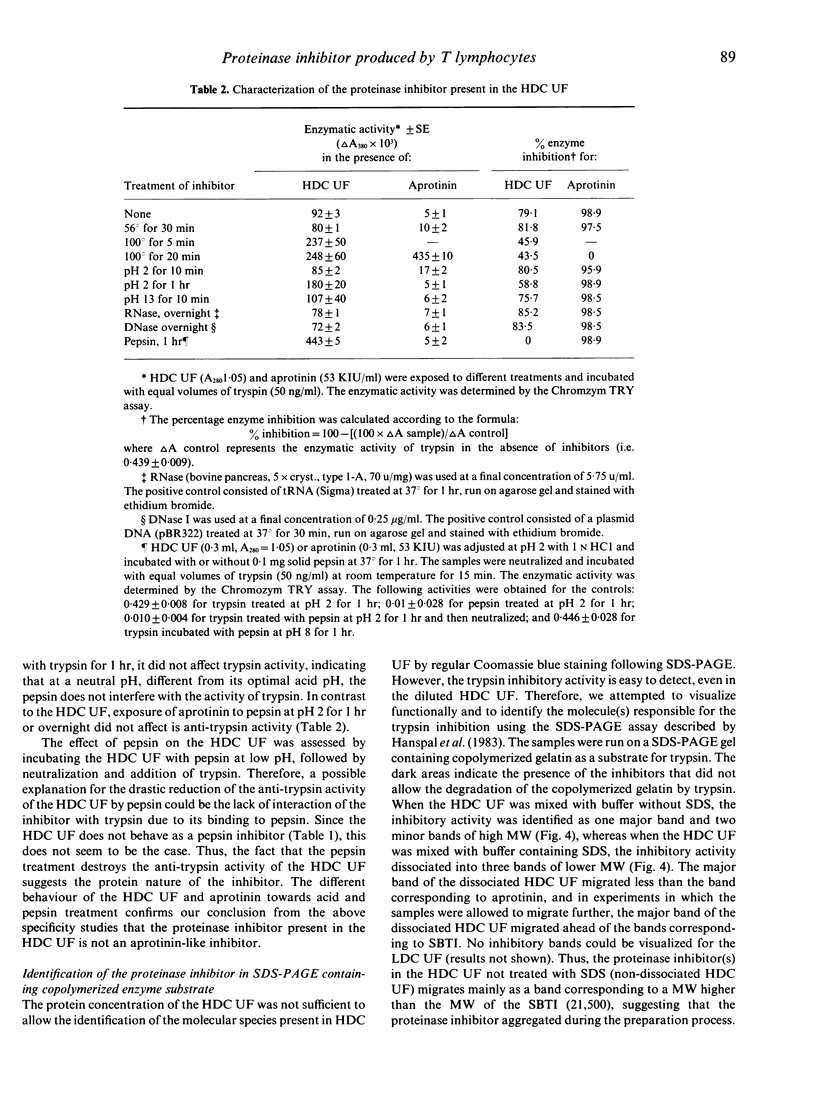
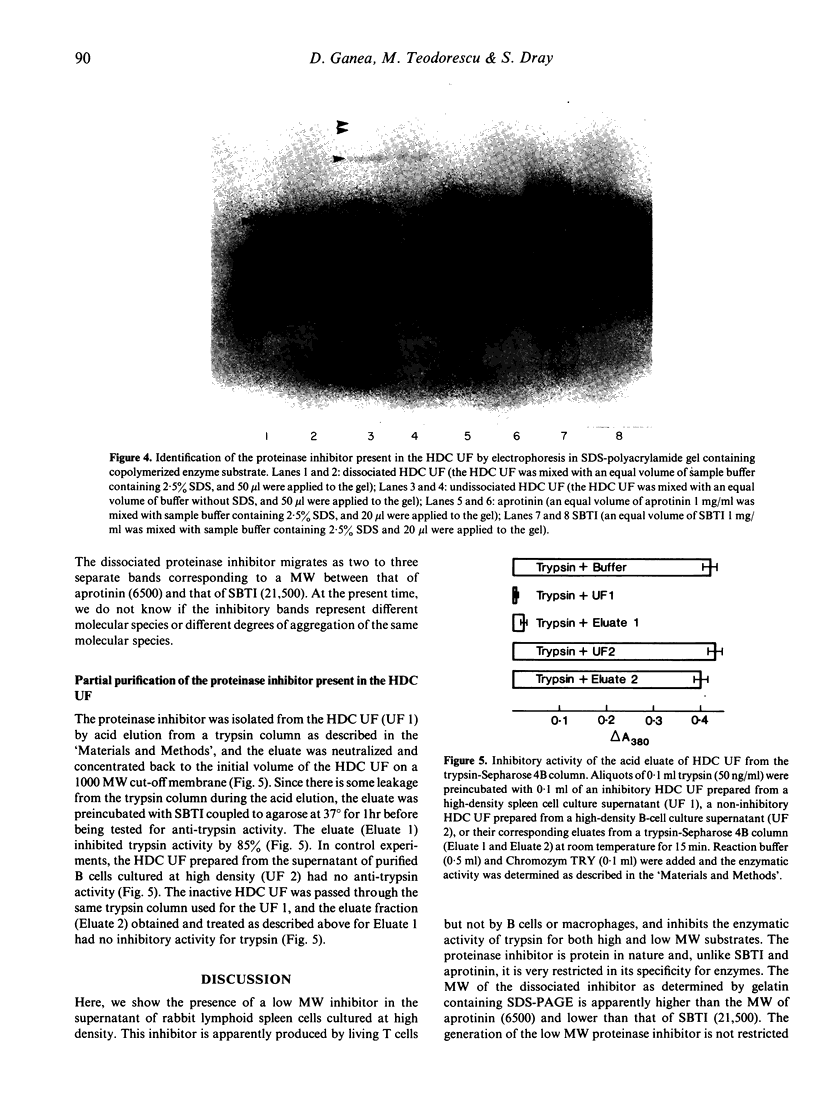
A low molecular weight (MW) proteinase inhibitor, between 6500 and 21,500 MW, appeared in the supernatant of rabbit spleen cells cultured at high density for 24 hr. The inhibitor inhibited the enzymatic activity of trypsin for both a high MW natural substrate, fibrinogen, and for a low MW artificial substrate, Chromozym TRY. The low MW proteinase inhibitor is protein in nature and is different, in terms of specificity for enzymes, MW and sensitivity to different physical or chemical treatments, from aprotinin, a low MW proteinase inhibitor (6500 MW) of bovine origin, and from the soybean trypsin inhibitor, a relatively high MW proteinase inhibitor (21,500 MW). The inhibitor was found in the supernatant of purified T cells but not B cells, and its production was increased in the presence of an optimal concentration of Con A. The possibility that this proteinase inhibitor has a role in the regulation of trypsin-like proteinases involved to the immune response remains to be investigated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J., Rattray S., Scott G. K. Preliminary evidence that thrombin may mimic a naturally occurring proteinase in cultured cells. Biosci Rep. 1981 Nov;1(11):881–884. doi: 10.1007/BF01114822. [DOI] [PubMed] [Google Scholar]
- Arnon R. The reaction of papain with antipapain. Immunochemistry. 1965 Jun;2(2):107–114. doi: 10.1016/0019-2791(65)90012-1. [DOI] [PubMed] [Google Scholar]
- Becker R. S., Draper L. R. Spontaneous helper factor production by nonadherent rabbit lymphoid cells and its feedback regulation by adherent cells. Cell Immunol. 1983 Sep;80(2):374–391. doi: 10.1016/0008-8749(83)90125-9. [DOI] [PubMed] [Google Scholar]
- Bieth J., Spiess B., Wermuth C. G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med. 1974 Dec;11(4):350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- Bromke B. J., Kueppers F. The major urinary protease inhibitor: simplified purification and characterization. Biochem Med. 1982 Feb;27(1):56–67. doi: 10.1016/0006-2944(82)90008-4. [DOI] [PubMed] [Google Scholar]
- Cechová D. Trypsin inhibitor from cow colostrum. Methods Enzymol. 1976;45:806–813. doi: 10.1016/s0076-6879(76)45074-7. [DOI] [PubMed] [Google Scholar]
- Cechová D. Trypsin inhibitor from cow colostrum. Methods Enzymol. 1976;45:806–813. doi: 10.1016/s0076-6879(76)45074-7. [DOI] [PubMed] [Google Scholar]
- Chang J. L., Ganea D., Dray S., Teodorescu M. An alpha2-macroglobulin associated factor produced by T lymphocytes which provides polyclonal stimulation of B lymphocytes to maintain the turnover of their surface Ig. Immunology. 1981 Dec;44(4):745–754. [PMC free article] [PubMed] [Google Scholar]
- Chang J. L., Ganea D., Dray S., Teodorescu M. Blast transformation of B cells induced by an alpha-macroglobulin-associated lymphokine produced in crowded lymphoid cell cultures. J Immunol. 1983 Jan;130(1):267–273. [PubMed] [Google Scholar]
- Fritz H., Kruck J., Rüsse I., Liebich H. G. Immunofluorescence studies indicate that the basic trypsin-kallikrein-inhibitor of bovine organs (Trasylol) originates from mast cells. Hoppe Seylers Z Physiol Chem. 1979 Mar;360(3):437–444. doi: 10.1515/bchm2.1979.360.1.437. [DOI] [PubMed] [Google Scholar]
- Fritz H., Tschesche H., Fink E. Proteinase inhibitors from boar seminal plasma. Methods Enzymol. 1976;45:834–847. doi: 10.1016/s0076-6879(76)45077-2. [DOI] [PubMed] [Google Scholar]
- Ganea D., Teodorescu M., Dray S. Allogeneic lymphocyte stimulation in rabbits: induction of a low MW inhibitor for trypsin and for a concurrently induced alpha-macroglobulin-proteinase complex. Immunology. 1985 Jul;55(3):437–446. [PMC free article] [PubMed] [Google Scholar]
- Glenn K. C., Cunningham D. D. Thrombin-stimulated cell division involves proteolysis of its cell surface receptor. Nature. 1979 Apr 19;278(5706):711–714. doi: 10.1038/278711a0. [DOI] [PubMed] [Google Scholar]
- Hanspal J. S., Bushell G. R., Ghosh P. Detection of protease inhibitors using substrate-containing sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1983 Jul 15;132(2):288–293. doi: 10.1016/0003-2697(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Hart D. A., Streilein J. S. Stimulation of a subpopulation of hamster lymphoid cells by trypsin and chymotrypsin. Exp Cell Res. 1976 Oct 15;102(2):246–252. doi: 10.1016/0014-4827(76)90039-2. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Iwata M., Munoz J. J., Ishizaka K. Modulation of the biologic activities of IgE-binding factor. IV. Identification of glycosylation-enhancing factor as a kallikrein-like enzyme. J Immunol. 1983 Oct;131(4):1954–1960. [PubMed] [Google Scholar]
- Kaplan J. G., Bona C. Proteases as mitogens. The Effect of trypsin and pronase on mouse and human lymphocytes. Exp Cell Res. 1974 Oct;88(2):388–394. doi: 10.1016/0014-4827(74)90257-2. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Kikutani H., Nishizawa Y., Sakaguchi N., Yamamura Y. Involvement of anti-Ig-activated serine protease in the generation of cytoplasmic factor(s) that are responsible for the transmission of Ig-receptor-mediated signals. J Immunol. 1979 Oct;123(4):1504–1510. [PubMed] [Google Scholar]
- Ku G. S., Quigley J. P., Sultzer B. M. The inhibition of the mitogenic stimulation of B lymphocytes by a serine protease inhibitor: commitment to proliferation correlates with an enhanced expression of a cell-associated arginine-specific serine enzyme. J Immunol. 1983 Nov;131(5):2494–2499. [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Lewis G. P. Immunoregulatory activity of metabolites of arachidonic acid and their role in inflammation. Br Med Bull. 1983 Jul;39(3):243–248. doi: 10.1093/oxfordjournals.bmb.a071827. [DOI] [PubMed] [Google Scholar]
- Liao Z., Grimshaw R. S., Rosenstreich D. L. Identification of a specific interleukin 1 inhibitor in the urine of febrile patients. J Exp Med. 1984 Jan 1;159(1):126–136. doi: 10.1084/jem.159.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard J., Favreau C. Rôle immunitaire de la plasmine. II. -- La plasmine modifie la réponse anticorps primaire des cultures de cellules spléniques. Ann Immunol (Paris) 1977 Nov-Dec;128(6):999–1013. [PubMed] [Google Scholar]
- Miki Y., Kishi H., Muraguchi A., Kishimoto S., Yamamura Y., Kishimoto T. The requirement for esterase activation in T cell replacing factor (TRF)-induced IgG production in a human B blastoid cell line. J Immunol. 1982 Feb;128(2):675–678. [PubMed] [Google Scholar]
- Rogers T. J., Campbell L., Calhoun K., Nowowiejski I., Webb D. R. Suppression of B-cell and T-cell responses by the prostaglandin-induced T-cell-derived suppressor (PITS). I. Analysis of the PITS beta factor. Cell Immunol. 1982 Jan 15;66(2):269–276. doi: 10.1016/0008-8749(82)90178-2. [DOI] [PubMed] [Google Scholar]
- Rogers T. J., Nowowiejski I., Webb D. R. Partial characterization of a prostaglandin-induced suppressor factor. Cell Immunol. 1980 Mar 1;50(1):82–93. doi: 10.1016/0008-8749(80)90008-8. [DOI] [PubMed] [Google Scholar]
- Spieker-Polet H., Hagen K., Teodorescu M. The role of intercellular contacts in the activation of B lymphocytes by anti-immunoglobulin antibodies. J Immunol. 1985 May;134(5):2827–2834. [PubMed] [Google Scholar]
- Takasaki S. I., Kasai K. I., Ishii S. I. Comparison of the catalytic properties of thrombin and trypsin by kinetic analysis on the basis of active enzyme concentration. J Biochem. 1975 Dec;78(6):1275–1285. doi: 10.1093/oxfordjournals.jbchem.a131025. [DOI] [PubMed] [Google Scholar]
- Teodorescu M., Buchholz D. M., Dray S. Maintenance of lymphocyte surface Ig by mitogen stimulation in vitro. J Immunol. 1975 Dec;115(6):1584–1586. [PubMed] [Google Scholar]
- Vischer T. L. Stimulation of mouse B lymphocytes by trypsin. J Immunol. 1974 Jul;113(1):58–62. [PubMed] [Google Scholar]



