Abstract
The ability of Entamoeba histolytica to kill and phagocytose host cells correlates with parasite virulence. This study addressed the role of apoptotic cell killing and host cell phosphatidylserine exposure in the subsequent phagocytosis of Jurkat T cells by E. histolytica. Ingested host cells were apoptotic, as evidenced by the activation of caspase 3 in 88% ± 3% (mean and standard deviation [SD] of the mean) of Jurkat cells engulfed by E. histolytica; ingested cells without detectable active caspase 3 were already disrupted and partially digested. That apoptotic cell killing preceded phagocytosis was supported by the demonstration that a higher percentage of amebae ingested apoptotic cells than ingested healthy cells (62% ± 7% versus 30% ± 9%, respectively [mean and SD]) (P = 0.008). E. histolytica also ingested apoptotic Jurkat cells more rapidly than necrotic control cells (8.5% ± 0.4% versus 3.5% ± 0.7%, respectively [mean and SD]) (P < 0.001). The inhibition of amebic cytotoxicity with d-galactose (which blocks the amebic Gal/GalNAc lectin) blocked the phagocytosis of healthy cells by greater than 80%, providing further evidence that apoptosis preceded engulfment. In contrast, d-galactose blocked the phagocytosis of already apoptotic cells by only 40%, implicating an additional host ligand (besides d-galactose) in amebic engulfment of apoptotic cells. The most characteristic surface change on apoptotic cells is phosphatidylserine exposure. Consistent with a role for host cell phosphatidylserine exposure in amebic ingestion of killed cells, Jurkat cell phosphatidylserine was exposed during incubation with E. histolytica (27% ± 1% [mean and SD] specific increase at 30 min) (the P value versus the control was 0.0003). Approximately 50% more amebae ingested viable Jurkat cells expressing phosphatidylserine on the outer leaflet of the plasma membrane than ingested control cells (30.3% ± 2.2% versus 19.8% ± 1.9%, respectively [mean and SD]) (P = 0.003). By analogy with phagocytic clearance during apoptosis in metazoans, amebic apoptotic host cell killing followed by phagocytosis may limit inflammation and enable amebae to evade the host immune response.
Entamoeba histolytica, the protozoan parasite that causes amebic colitis and liver abscesses, is characterized clinically by its ability to cause indolent infection and pathologically by only moderate inflammation during chronic infection, despite extraordinary tissue destruction (1, 6, 19, 20). Amebic cytotoxicity and phagocytic ability both correlate with parasite virulence (21, 23, 24, 26, 31, 35). Indeed, the observation of ingested host cells is the only characteristic on light microscopy that distinguishes E. histolytica from the nonpathogenic intestinal commensal organism Entamoeba dispar (16).
The specific roles of host cell killing and phagocytosis in the pathogenesis of invasive amebiasis remain unknown. Amebic host cell killing is contact dependent and is mediated by an amebic Gal/GalNAc adherence lectin, but the exact mechanism of cell death remains controversial (25-27). Huston et al. (18) recently demonstrated rapid caspase 3-dependent apoptosis of Jurkat leukemia T cells killed by amebic trophozoites in vitro, while Berninghausen and Leippe stressed a necrotic mechanism of cell death (4). During cecal invasion in mice, amebic trophozoites are readily seen with ingested intact apoptotic cells (as determined by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) (18). Similarly, extensive apoptotic cell death occurs during mouse liver abscess formation. The ability of the nonspecific peptidic caspase inhibitor zVAD-fmk to block both apoptosis and liver abscess formation is consistent with a requirement for apoptotic cell killing for pathogenesis (34, 38). In multicellular organisms, phagocytosis is the final step in the apoptotic pathway and serves to limit inflammation by preventing spillage of toxic intracellular contents of dead cells (11, 32). Amebic ingestion of killed cells could similarly limit the host inflammatory response and enable E. histolytica to establish a persistent infection. Here we tested the hypothesis that the apoptotic phenotype of cells killed by E. histolytica facilitates their ingestion and examined the role of host cell phosphatidylserine exposure during amebic cell killing in subsequent phagocytosis by amebae.
MATERIALS AND METHODS
Chemicals and reagents.
Actinomycin d, d-mannose, d-galactose, and fluorescein isothiocyanate (FITC)-dextran (average molecular mass, 40 kDa) were purchased from Sigma (St. Louis, Mo.). The caspase 3 inhibitor Ac-DEVD-CHO was purchased from Calbiochem (San Diego, Calif.). The fluorescent dyes 5 (and 6)-carboxytetramethylrhodamine succinimidyl ester (TAMRA), 5 (and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE), and 5 (and 6)-4-chloromethyl-benzoylaminotetramethylrhodamine (CMTMR) were purchased from Molecular Probes (Eugene, Oreg.). Annexin V-FITC and FITC-conjugated rabbit anti-active caspase 3 monoclonal antibodies were purchased from PharMingen (San Diego, Calif.). The following phospholipids were purchased from Avanti Polar Lipids (Alabaster, Ala.): l-α-phosphatidylcholine, 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-l-serine, l-α-phosphatidic acid, and l-α-phosphatidylethanolamine.
Cell lines, culturing, and triggering of cell death.
E. histolytica trophozoites (HM1:IMSS) were grown axenically in TYI-S-33 (Trypticase-yeast extract-iron-serum) medium supplemented with 100 U of penicillin/ml and 100 μg of streptomycin sulfate/ml at 37°C (10). Trophozoites were harvested for experiments during log-phase growth by incubation on ice for 10 min, centrifugation at 200 × g and 4°C for 5 min, and resuspension in medium 199 (Gibco BRL, Grand Island, N.Y.) supplemented with 5.7 mM cysteine, 25 mM HEPES, and 0.5% bovine serum albumin at pH 6.8 (M199s medium). In some experiments, amebae were pretreated with 10 mM NH4Cl (14 h), which partially blocks amebic killing of host cells (29).
The human leukemia T-cell line Jurkat-E6-1 (American Type Culture Collection, Manassas, Va.) was grown in complete medium (RPMI 1640 medium (Gibco BRL) supplemented with 10% fetal bovine serum, 100 U of penicillin/ml, and 100 μg of streptomycin sulfate/ml). Prior to use, cultures were enriched for viable Jurkat cells by centrifugation at 800 × g for 10 min at room temperature through Ficoll-Paque PLUS (Amersham Biosciences, Piscataway, N.J.) as previously described (5). Jurkat cell apoptosis was induced by treatment with actinomycin D (5 μg/ml, 14 h). Necrotic cell death was induced by incubation at 55°C for 15 min (9). Each of these treatments consistently resulted in greater than 85% cell death, as determined by changes in forward and side scatter characteristics on flow cytometry (data not shown).
Fluorescent labeling of cells.
Prior to induction of cell death, target cells were fluorescently labeled with one of three dyes: TAMRA (red), CMTMR (red), or CFSE (green). Jurkat cells were labeled red by incubation at 37°C for 20 to 25 min in phosphate-buffered saline (PBS) containing either 47 μM TAMRA or 120 μM CMTMR. Alternatively, Jurkat cells were labeled green by incubation at 37°C for 10 min with 5 μM CFSE in PBS. Unbound dye was quenched in each case by incubation with an equal volume of fetal bovine serum at 37°C for 25 min, and cells were washed twice with complete medium before use.
Insertion of phosphatidylserine into the Jurkat cell plasma membrane outer leaflet.
Small unilamellar liposomes were prepared by sonication of phospholipids as previously described (12, 14). Briefly, lipids suspended in chloroform were added to glass tubes, the chloroform was evaporated under N2 gas, and the lipids were resuspended in PBS by vortexing. The lipid suspensions were then sonicated until they became opalescent. Phosphatidylcholine liposomes contained only phosphatidylcholine. Other liposomes contained 50 mol% phosphatidylcholine and 50 mol% phosphatidylserine, phosphatidic acid, or phosphatidylethanolamine. After preparation of liposomes, 30 μmol of total lipid was added to 106 viable Jurkat cells in 1.5 ml of PBS, and the mixture was incubated at 37°C for 30 min while tumbling end-over-end. The cells were then washed twice with PBS and used immediately.
Adherence assay.
E. histolytica adherence was assayed with a rosette formation assay (27). Amebae (105) and healthy or apoptotic (actinomycin D-treated) Jurkat cells (4 × 105) were resuspended in 1 ml of M199s medium, centrifuged (200 × g, 5 min, 4°C), and incubated for 30 min on ice. After incubation, the supernatant was poured off, the pellet was suspended with a Pasteur pipette, and 1 drop was placed on a hemacytometer. Adherent amebae were defined as amebae with three or more bound Jurkat cells, and data were normalized to those for control samples (healthy Jurkat cells without carbohydrate) to enable combining the results of three experiments.
Annexin V binding assay.
Jurkat cell surface exposure of phosphatidylserine (due to either lipid reconstitution or apoptosis) was quantitated with annexin V-FITC binding and flow cytometry. The cells were stained with annexin V as recommended by the manufacturer (PharMingen) and analyzed by using a FACScan cytometer and CellQuest 3.3 software (Becton Dickinson, Franklin Lakes, N.J.). For experiments measuring Jurkat cell phosphatidylserine exposure during incubation with E. histolytica trophozoites, amebae and Jurkat cells were centrifuged (200 × g, 5 min, 4°C) and incubated (E. histolytica/Jurkat cell ratio, 1:3; 37°C) for various times in M199s medium. Ameba-host cell rosettes were then disrupted by washing with cold PBS supplemented with 110 mM d-galactose (which blocks Gal/GalNAc lectin-mediated amebic adherence) and stained with annexin V-FITC. Jurkat cell uptake of FITC-dextran (average molecular mass, 40 kDa) was used as a control to ensure that annexin V-FITC (molecular mass, 35 to 36 kDa) staining represented exposure of phosphatidylserine on the outer leaflet of the plasma membrane and not internal staining of Jurkat cells due to membrane disruption (7).
Phagocytosis assays.
Phagocytosis was assayed by either microscopy or flow cytometry. Confocal microscopy was used to distinguish ingested from adherent Jurkat cells. Amebae were scored as positive for phagocytosis if they contained one or more ingested Jurkat cells. Results were expressed as a phagocytic index, which was the percentage of amebic trophozoites that had phagocytosed Jurkat cells multiplied by the average number of Jurkat cells per ameba (14).
The flow cytometry assay for amebic ingestion of host cells was adapted from that of Hess et al. (17). Fluorescently labeled Jurkat cells were mixed with amebic trophozoites in M199s medium, and the mixture was centrifuged (200 × g, 5 min, 4°C) and incubated at 37°C for 10 to 20 min. Following incubation, ameba-host cell rosettes were disrupted by resuspension in 110 mM d-galactose in ice-cold PBS. Cells were then fixed with 3% paraformaldehyde and analyzed by flow cytometry. Amebae and Jurkat cells were distinguished on the basis of differences in forward and side scatter characteristics and background fluorescence. E. histolytica ingestion of fluorescent Jurkat cells was measured as increased amebic red and green fluorescence and quantitated as the percentage of amebae with fluorescence above background levels.
Confocal microscopy.
E. histolytica trophozoites (5 × 105) were allowed to adhere to acetone-washed glass coverslips in 24-well tissue culture plates by incubation in TYI-S-33 medium at 37°C for 20 to 30 min and then were washed twice with warm PBS. TAMRA-labeled healthy or apoptotic Jurkat cells in M199s medium were added at various ameba/Jurkat cell ratios. To synchronize phagocytosis, Jurkat cells were centrifuged (200 × g, 5 min) onto amebae by using a plate spinner, and the plates were then incubated at 37°C for various times. Following incubation, the cells were washed with warm PBS, fixed with 3% paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS, blocked with 20% goat serum-5% bovine serum albumin in PBS (1 h, 37°C), and stained. FITC-conjugated rabbit anti-active caspase 3 monoclonal antibodies were used according to the manufacturer's instructions to detect Jurkat cell caspase 3 activation. Amebic Gal/GalNAc lectin was stained with rabbit antilectin polyclonal antibodies and a FITC-conjugated anti-rabbit mouse antibody (Sigma). After staining, the coverslips were washed twice with PBS, inverted onto Gel Mount (Biomeda, Foster City, Calif.) on glass slides, and examined by using a Zeiss LSM 410 laser scanning confocal microscope equipped with an argon-krypton laser.
Statistics.
Data were expressed as the mean and standard deviation (SD) of the mean. Significance was determined by using the unpaired Student t test.
RESULTS
Jurkat cells ingested by E. histolytica were apoptotic.
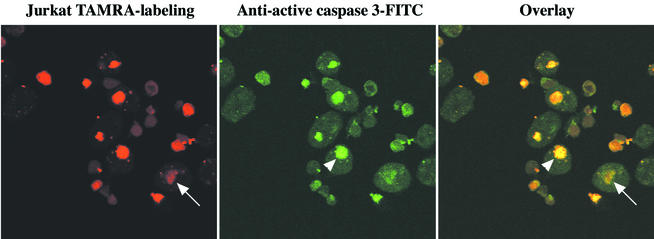
E. histolytica kills Jurkat leukemia T cells in vitro by induction of caspase 3-dependent apoptosis (18). To investigate whether apoptotic host cell killing by amebae facilitated amebic phagocytosis (in a fashion analogous to phagocytosis of apoptotic cells in metazoans), we first determined whether lymphocytes ingested by E. histolytica were apoptotic. Healthy TAMRA-labeled Jurkat cells (red) were mixed with E. histolytica trophozoites (E. histolytica/Jurkat cell ratio, 1:2), stained with FITC-conjugated anti-active caspase 3 monoclonal antibodies (green), and examined by confocal microscopy (Fig. 1). One hundred ingested cells per slide on three slides were scored positive or negative for apoptosis, and definite caspase 3 activation was observed in 88% ± 3% of ingested cells (mean and SD) (Fig. 1, green channel and overlay). The 12% ± 3% of phagocytosed cells without clear caspase activation appeared degraded (Fig. 1, red channel and overlay), suggesting that caspase activity may not have been detected because of a loss of cellular contents. Many uningested cells were nonapoptotic, staining for active caspase 3 at background levels and serving as an internal control. Note that the Jurkat cells with active caspase 3 appeared green but also appeared more red than viable cells because the fluorescein emission (λmax, 525 nm) excited the TAMRA label (λmax, 553 nm; 47% of the maximal excitation at a λ value of 525 nm). We concluded that caspase 3 activation preceded phagocytosis.
FIG. 1.
Confocal microscopy of active caspase 3 in Jurkat cells ingested by E. histolytica. Healthy TAMRA-labeled Jurkat cells (red) were centrifuged onto E. histolytica trophozoites (E. histolytica/Jurkat cell ratio, 1:2). Following 20 min of incubation (37°C), slides were fixed and stained for active caspase 3 with FITC-conjugated rabbit anti-active caspase 3 monoclonal antibodies (green). Arrowheads indicate a phagocytosed Jurkat cell with active caspase 3 (yellow). Arrows indicate a degraded phagocytosed cell. Original magnification, ×800.
E. histolytica ingested apoptotic Jurkat cells more efficiently than healthy or necrotic cells.
To delineate further E. histolytica phagocytosis from adherence and cytolysis, we compared the abilities of amebae to phagocytose healthy versus already apoptotic Jurkat cells (killed by preincubation with actinomycin D). The phagocytic event was partially isolated from host cell killing by pretreatment of amebae with 10 mM NH4Cl. This treatment inhibits amebic host cell killing by approximately 40%, as measured both by trypan blue staining (necrosis) and by release of 3H-thymidine (DNA fragmentation and apoptosis) (29). Healthy or apoptotic Jurkat cells were labeled with TAMRA (red) and mixed with E. histolytica trophozoites (E. histolytica/Jurkat cell ratio, 1:4; 37°C, 10 min). Amebae were then stained with rabbit anti-Gal/GalNAc lectin polyclonal antibodies and a FITC-conjugated anti-rabbit mouse antibody (green).
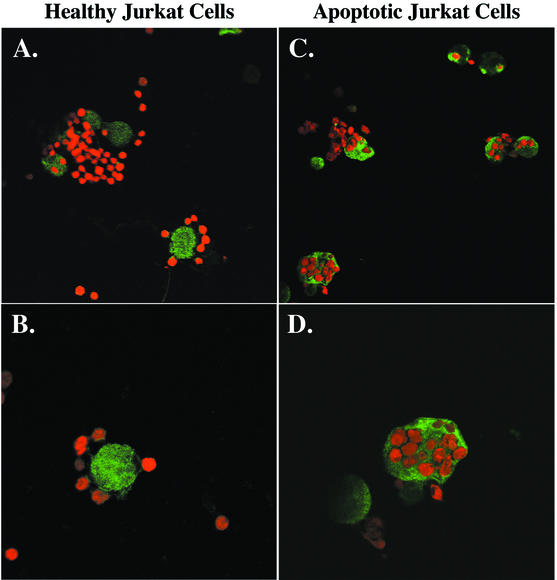
Healthy Jurkat cells were found adherent to but infrequently ingested by NH4Cl-treated amebae (Fig. 2A and B). In contrast, actinomycin D-killed Jurkat cells were predominantly phagocytosed by E. histolytica (Fig. 2C and D). Phagocytosis was quantitated by counting the number of ingested Jurkat cells per ameba by using confocal microscopy (Table 1). A higher percentage of amebae incubated with apoptotic than with healthy Jurkat cells ingested at least one cell (62% ± 7% versus 30% ± 9%, respectively [mean and SD, n = 3]) (P = 0.008). In addition, the number of apoptotic cells ingested per ameba was higher (2.3 ± 0.3 versus 1.3 ± 0.1 Jurkat cells, respectively [mean and SD]) (P < 0.003), resulting in a phagocytic index more than threefold higher for the ingestion of apoptotic cells (142 ± 30 versus 39 ± 11, respectively [mean and SD]) (P = 0.005).
FIG. 2.
Confocal microscopy of the interaction of healthy and previously killed Jurkat cells with NH4Cl-treated E. histolytica trophozoites. TAMRA-labeled healthy or apoptotic Jurkat cells (red) were centrifuged onto NH4Cl-pretreated E. histolytica trophozoites (E. histolytica/Jurkat cell ratio, 1:4). Following 10 min of incubation (37°C), unbound cells were washed away, and remaining cells were fixed and stained for E. histolytica Gal/GalNAc lectin with rabbit antilectin polyclonal antibodies and FITC-conjugated anti-rabbit mouse antibody (green). Original magnifications, A and C, ×400; B and D, ×800.
TABLE 1.
Ingestion of healthy Jurkat cells and Jurkat cells killed with actinomycin D
| Jurkat cells | % of E. histolytica trophozoites with ingested Jurkat cellsb | No. of Jurkat cells ingested/positive trophozoitec | Phagocytic indexd |
|---|---|---|---|
| Healthy | 30 ± 9 | 1.3 ± 0.1 | 39 ± 11 |
| Apoptotic | 62 ± 7 | 2.3 ± 0.3 | 142 ± 30 |
Phagocytosis was determined by confocal microscopy to count the number of ingested Jurkat cells per trophozoite. Three slides per condition and 100 trophozoites per slide were evaluated. Data are reported as means and SDs. P values were determined by a two-tailed t test for healthy and apoptotic cells.
P = 0.008.
P = 0.003.
P = 0.005.
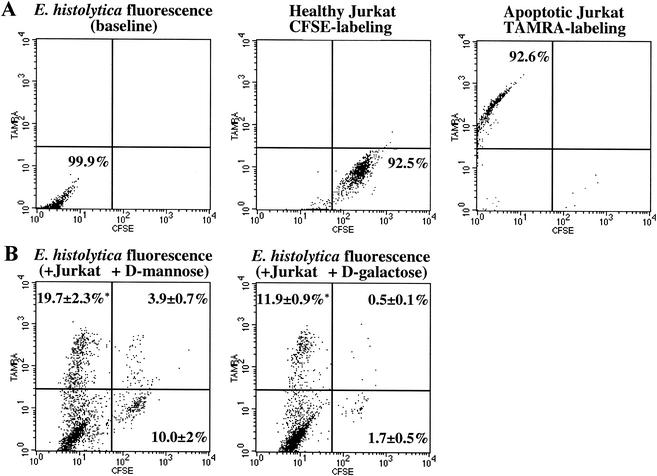
To independently test whether E. histolytica ingested apoptotic cells more efficiently than healthy cells, we developed a rapid, quantitative flow cytometry assay for amebic ingestion of host cells. The method measured E. histolytica ingestion of apoptotic Jurkat cells labeled with TAMRA and healthy Jurkat cells labeled with CFSE. The apoptotic and healthy cell populations were mixed at a 1:1 ratio and incubated with NH4Cl-treated amebic trophozoites in the presence of either 50 mM d-galactose to block Gal/GalNAc lectin-mediated adherence or d-mannose as an osmotic control (E. histolytica/healthy Jurkat/apoptotic Jurkat cell ratio, 1:1:1; 37°C, 10 min). Following incubation, amebic phagocytosis was quantitated by using two-color flow cytometry as described above.
The labeled apoptotic and healthy Jurkat cell populations were easily separated by their red and green fluorescence (Fig. 3A). The large size and vesicularity of E. histolytica enabled the inclusion of greater than 90% of amebae in the analysis but the exclusion of greater than 95% of uningested Jurkat cells (data not shown). The percentages of amebae that had ingested TAMRA (apoptotic)- and/or CFSE (healthy)-labeled Jurkat cells were determined by analyzing 10,000 amebae per sample. Consistent with the confocal microscopy results, the fluorescence-activated cell sorting (FACS) assay demonstrated greater phagocytosis of apoptotic than of healthy cells by NH4Cl-treated amebae (19.7% ± 2.3% versus 10.0% ± 2% positive amebae, respectively [mean and SD, n = 3]) (P < 0.006) (Fig. 3B, left). In the presence of galactose at a dose that nearly completely blocks amebic adherence and cell killing, six times as many amebae ingested apoptotic cells as ingested healthy cells (11.9% ± 0.9% versus 1.7% ± 0.5%, respectively [mean and SD, n = 3]) (P < 0.006) (Fig. 3B, right). Importantly, inhibition of the Gal/GalNAc lectin reduced the phagocytosis of healthy Jurkat cells by greater than 80% but reduced the phagocytosis of killed Jurkat cells by only 40%. This result indicated that, in addition to the lectin, other amebic receptors that recognize surface changes on apoptotic cells may participate in amebic phagocytosis. Interestingly, the inhibition of caspase 3 by pretreatment of Jurkat cells with the caspase 3 inhibitor Ac-DEVD-CHO under conditions (100 μM, 37°C, 1 h) previously shown to block host cell DNA fragmentation during incubation with amebic trophozoites did not reduce amebic phagocytosis (data not shown) (18). Therefore, if surface changes that triggered amebic phagocytosis occurred during apoptotic cell killing by amebae, then they occurred independently or upstream of caspase 3 activation.
FIG. 3.
FACS analysis showing phagocytosis of healthy and apoptotic Jurkat cells by NH4Cl-treated E. histolytica trophozoites. Amebic phagocytosis was measured by flow cytometry following coincubation of amebae with CFSE-labeled healthy (green) and TAMRA-labeled apoptotic (red) Jurkat cells (E. histolytica-healthy Jurkat-apoptotic Jurkat cell ratio, 1:1:1; 37°C, 10 min). (A) Two-color dot plots showing TAMRA and CFSE fluorescence of amebae and healthy or apoptotic Jurkat cell populations. (B) Representative two-color dot plots showing amebic fluorescence following incubation with both labeled Jurkat cell populations in the presence of 50 mM d-mannose (osmotic control, left) or 50 mM d-galactose (right). Values are percentages of amebae positive for phagocytosis of apoptotic (upper left), healthy (lower right), or both apoptotic and healthy (upper right) cells (mean and SD, n = 3). Asterisks indicate a P value of <0.006 for comparisons with ingestion of healthy cells.
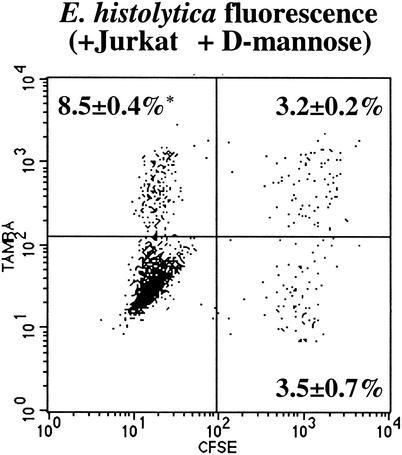
We were interested in determining whether E. histolytica host cell phagocytosis was specific for apoptotic cells or simply was a result of cell death. Surface changes on both apoptotic and necrotic cells are recognized by macrophages in multicellular organisms, although via distinct and noncompeting mechanisms (9). TAMRA-labeled apoptotic Jurkat cells and CFSE-labeled necrotic Jurkat cells were mixed and fed to amebic trophozoites (E. histolytica/apoptotic Jurkat/necrotic Jurkat cell ratio, 1:1:1; 37°C, 10 min). Twice as many NH4Cl-treated amebae ingested apoptotic as ingested necrotic Jurkat cells (8.5% ± 0.4% versus 3.5% ± 0.7%, respectively [mean and SD, n = 3]) (P < 0.001) (Fig. 4), suggesting specific recognition and uptake of apoptotic cells.
FIG. 4.
FACS analysis showing phagocytosis of necrotic and apoptotic Jurkat cells by NH4Cl-treated E. histolytica trophozoites. Amebic phagocytosis of CFSE-labeled necrotic (green) and TAMRA-labeled apoptotic (red) Jurkat cells (E. histolytica-necrotic Jurkat-apoptotic Jurkat cell ratio, 1:1:1; 37°C, 10 min) was measured by flow cytometry. A representative two-color dot plot showing amebic fluorescence following incubation with both labeled Jurkat cell populations is shown. Values are percentages of amebae positive for phagocytosis of apoptotic (upper left), necrotic (lower right), or both apoptotic and necrotic (upper right) cells (mean and SD, n = 3). The asterisk indicates a P value of <0.001 for a comparison with ingestion of necrotic cells.
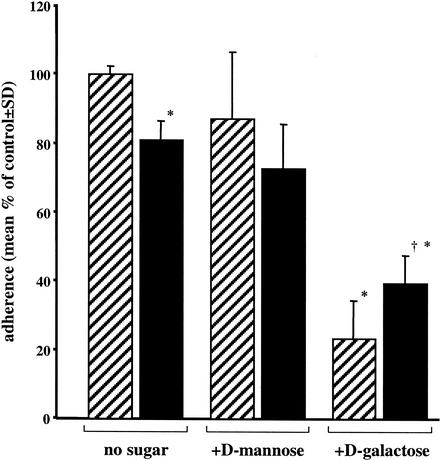
Enhanced amebic phagocytosis of apoptotic Jurkat cells was not due to increased adherence.
That E. histolytica ingested apoptotic cells more efficiently than nonapoptotic cells suggested that increased phagocytosis resulted from enhanced amebic adherence to apoptotic cells and/or from an ability of apoptotic cells to trigger ingestion via unknown amebic receptors. We quantitated amebic adherence to healthy and apoptotic Jurkat cells by using a rosette formation assay (Fig. 5). Surprisingly, approximately 20% fewer amebae adhered to apoptotic Jurkat cells than to healthy control cells (81% ± 6% of adherent amebae [normalized to the control] [mean and SD, n = 9]) (P < 0.01). This result indicated that the increased phagocytosis of apoptotic cells by amebae was not simply due to increased amebic adherence. Importantly, the adherence of E. histolytica to apoptotic cells was only partially inhibited by 50 mM d-galactose (51% ± 10% versus 77% ± 11% inhibition for apoptotic and healthy cells, respectively [mean and SD, n = 9]) (P < 0.01). These data were consistent with the previously observed limited ability of d-galactose to inhibit the phagocytosis of already apoptotic cells (Fig. 3B) and further implicated an additional amebic receptor in the phagocytosis of apoptotic cells.
FIG. 5.
Adherence of E. histolytica trophozoites to healthy (cross-hatched bars) and apoptotic (black bars) Jurkat cells. Adherence was measured in the presence or absence of 50 mM d-mannose (osmotic control) or 50 mM d-galactose and expressed as the percentage of amebae adherent to healthy Jurkat cells in the absence of carbohydrate. An adherent ameba was defined as one having at least three adherent Jurkat cells (mean and SD, n = 9). Asterisks indicate a P value of <0.01 for comparisons with healthy cells without sugar; a dagger indicates a P value of <0.01 for a comparison with healthy cells plus d-galactose.
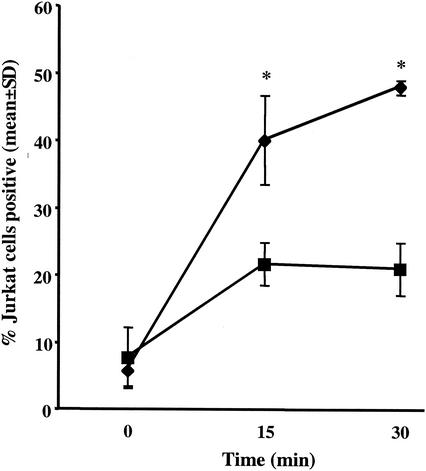
Jurkat cell phosphatidylserine was exposed during incubation with E. histolytica trophozoites.
The transfer of phosphatidylserine from the inner monolayer to the outer monolayer of the plasma membrane is the most characteristic surface change on apoptotic cells. The recognition and engulfment of apoptotic cells and senescent erythrocytes are mediated in part by phosphatidylserine receptors on macrophages (12, 14, 22). Furthermore, Bailey et al. previously demonstrated that phosphatidylserine-containing liposomes stimulate E. histolytica actin polymerization, a surrogate marker for phagocytosis (2). To determine whether exposure of phosphatidylserine on the plasma membrane outer leaflet of cells killed by E. histolytica could be the trigger for amebic ingestion of apoptotic cells, we first quantitated phosphatidylserine on Jurkat cells after interaction with amebic trophozoites by measuring annexin V-FITC binding by using flow cytometry (E. histolytica/Jurkat cell ratio, 1:3; 37°C). Jurkat cell phosphatidylserine exposure occurred rapidly during incubation with amebae, as was expected due to the apoptotic killing of these cells by E. histolytica (Fig. 6). To control for the potential leakage of annexin V into Jurkat cells, Jurkat cell staining with FITC-dextran (average molecular mass, 40 kDa), which has a molecular mass similar to that of annexin V-FITC (molecular mass, 35 to 36 kDa), was also measured (7). While annexin V-FITC staining in excess of FITC-dextran staining indicated specificity, it is important to note that FITC-dextran staining increased by approximately 13% during incubation with amebae, indicating significant membrane disruption in this subset of Jurkat cells.
FIG. 6.
Time course of Jurkat cell phosphatidylserine exposure during incubation with amebic trophozoites. Following incubation of healthy Jurkat cells with E. histolytica trophozoites for the indicated times (E. histolytica-Jurkat cell ratio, 1:3; 37°C), Jurkat cell phosphatidylserine exposure was measured as annexin V-FITC binding by flow cytometry. Jurkat cell staining with FITC-dextran (molecular mass similar to that of annexin V) was used to ensure that annexin V-FITC binding was specific for phosphatidylserine exposure on the outer leaflet of the plasma membrane and was not a result of membrane damage (mean and SD for FITC-positive Jurkat cells, n = 3). Asterisks indicate a P value of ≤0.01 for comparisons with the FITC-dextran control. Symbols: ♦, annexin V-FITC; ▪, FITC-dextran.
Expression of phosphatidylserine on the outer leaflet of the plasma membrane of Jurkat cells enhanced amebic phagocytosis.
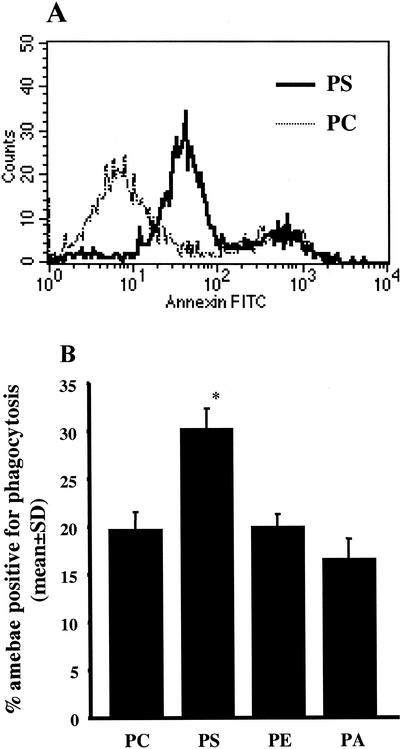
To directly test whether Jurkat cell phosphatidylserine expression contributed to amebic phagocytosis, phosphatidylserine was inserted into the outer monolayer of healthy Jurkat cells. This method was recently used by Fadok et al. to demonstrate enhanced uptake of phosphatidylserine-expressing Jurkat cells by macrophages (12). As measured by flow cytometry, Jurkat cells reconstituted with phosphatidylserine-containing liposomes bound more annexin V-FITC than cells reconstituted with phosphatidylcholine-containing liposomes (Fig. 7A). Jurkat cells reconstituted with phosphatidylethanolamine- and phosphatidic acid-containing liposomes bound annexin V-FITC at the level of the phosphatidylcholine control (data not shown for clarity of the overlay histogram). Of note, approximately 20% of Jurkat cells treated with each lipid began to undergo spontaneous apoptosis during treatment and therefore were stained with annexin V-FITC at high levels (Fig. 7A, rightmost peak, and data not shown).
FIG. 7.
E. histolytica phagocytosis of viable Jurkat cells that express phosphatidylserine on the outer leaflet of the plasma membrane via liposome transfer. CMTMR-labeled healthy Jurkat cells were coated with liposomes containing either phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE), or phosphatidic acid (PA) and incubated with NH4Cl-treated amebic trophozoites (E. histolytica/Jurkat cell ratio, 1:2; 37°C, 20 min) in the presence of 50 mM d-galactose. (A) Outer membrane PS expression on Jurkat cells coated with PS-containing liposomes. Liposome-coated Jurkat cells were labeled with annexin V-FITC and analyzed by flow cytometry. The histogram overlay shows annexin V-FITC binding by Jurkat cells incubated with PS- or PC-containing liposomes. (B) Amebic phagocytosis of lipid-coated viable Jurkat cells. Data are the percentages of amebae positive for phagocytosis of CMTMR-labeled Jurkat cells, as determined by flow cytometry (mean and SD, n = 3). An asterisk indicates a P value of ≤0.003 for comparisons with all other lipids.
E. histolytica phagocytosis of Jurkat cells coated with phosphatidylserine or control lipids was quantitated by using flow cytometry (E. histolytica/Jurkat cell ratio, 1:2; 37°C, 20 min) (Fig. 7B). Again, pretreatment of amebae with 10 mM NH4Cl and the addition of 50 mM d-galactose were used to isolate the phagocytic event from cell killing. Approximately 50% more amebae ingested phosphatidylserine-expressing Jurkat cells than ingested control cells reconstituted with phosphatidylcholine (30.3% ± 2.2% versus 19.8% ± 1.9% amebae positive for phagocytosis, respectively [mean and SD, n = 3]) (P = 0.003). In contrast, another negatively charged phospholipid, phosphatidic acid, did not increase phagocytosis. We concluded that the presence of phosphatidylserine on the outer leaflet was a relatively specific signal for amebic engulfment.
DISCUSSION
E. histolytica host cell killing and phagocytosis are central features of invasive amebiasis. Our study of the role that apoptotic host cell killing plays in amebic phagocytosis had three major conclusions: (i) E. histolytica apoptotic cell killing preceded phagocytosis; (ii) inhibition of the Gal/GalNAc lectin only partially inhibited amebic ingestion of and adherence to apoptotic cells, implicating another receptor in the recognition and clearance of killed cells; and (iii) host cell phosphatidylserine was exposed on the outer membrane of cells killed by E. histolytica, and the surface expression of phosphatidylserine enhanced amebic phagocytosis.
Several experiments indicated the specific recognition and uptake of apoptotic cells by E. histolytica. Caspase 3 activity was detected in virtually all intact host cells ingested by E. histolytica. Furthermore, it was possible to partially isolate cell killing from phagocytosis by pretreating amebae with NH4Cl, which blocks both amebic apoptotic and cytolytic killing of host cells. Under these conditions, E. histolytica ingested apoptotic cells more efficiently than nonapoptotic cells. Ongoing phagocytosis of healthy Jurkat cells was likely due to an incomplete blockade of amebic cell killing and to the ingestion of cells undergoing spontaneous apoptosis, since NH4Cl inhibits amebic cytotoxicity by only approximately 40% and approximately 10% of the Jurkat cells used in these experiments were undergoing spontaneous apoptosis in cultures (reference 29 and data not shown). The ingestion of healthy cells was nearly completely eliminated by the more complete inhibition of amebic cytotoxicity by d-galactose, which blocks the amebic Gal/GalNAc adherence lectin. Importantly, E. histolytica preferentially ingested apoptotic cells in direct competition with necrotic cells. Taken together, these data indicated that apoptotic killing of host cells by amebae preceded phagocytosis and that the trigger(s) for increased amebic phagocytosis was specific for apoptotic cells and was not simply a general feature of dying cells.
Several experiments implicated at least one amebic receptor in addition to the Gal/GalNAc lectin in the recognition and phagocytosis of apoptotic cells by amebae. The addition of d-galactose at a concentration sufficient to nearly completely block amebic cytotoxicity reduced the phagocytosis of viable cells by approximately 80% but reduced the phagocytosis of already apoptotic cells by only 40%. Furthermore, amebic ingestion of apoptotic cells was not simply a result of increased adherence (e.g., due to more galactose on the dying cells); in fact, approximately 20% fewer amebae adhered to apoptotic cells than to healthy control cells. In the presence of d-galactose, however, significantly more amebae adhered to apoptotic Jurkat cells than to healthy cells. It is important to note that d-galactose did substantially reduce both adherence to and phagocytosis of already apoptotic cells. We concluded that, while the Gal/GalNAc lectin participated in adherence to apoptotic cells during the phagocytic process, at least one additional amebic receptor with a limited role in adherence but the ability to trigger phagocytosis was involved.
The redistribution of phosphatidylserine from the inner to the outer leaflet of the plasma membrane is the most characteristic surface feature of apoptotic cells (12, 14). Therefore, the transfer of phosphatidylserine from the inner to the outer leaflet of the Jurkat cell plasma membrane was an expected result of apoptotic killing of Jurkat cells by E. histolytica, and the exposure of phosphatidylserine was confirmed by specific annexin V binding. A significant increase in FITC-dextran staining of Jurkat cells during incubation with amebic trophozoites also occurred and indicated membrane disruption in this subset of cells. The delay in FITC-dextran staining relative to annexin V-FITC staining suggested that membrane disruption might have followed apoptotic killing of these cells (i.e., secondary necrosis). Alternatively, this subset of cells might have been killed by necrosis.
The importance of phosphatidylserine exposure for E. histolytica engulfment of host cells was suggested by the work of Bailey et al., who previously demonstrated that liposomes containing phosphatidylserine or a synthetic negatively charged phospholipid, dicetyl phosphate, stimulate E. histolytica actin polymerization (2). To directly test the ability of host cell phosphatidylserine exposure to stimulate amebic phagocytosis, we quantitated amebic ingestion of viable Jurkat cells following reconstitution of the plasma membrane with phosphatidylserine or several control lipids. Approximately 50% more amebae ingested Jurkat cells expressing phosphatidylserine on the outer leaflet of the plasma membrane than ingested cells coated with phosphatidylcholine, phosphatidylethanolamine, or phosphatidic acid. Bailey et al. (2) suggested that the negative charge on the plasma membrane outer leaflet triggers amebic actin polymerization, so we were surprised to find that phosphatidic acid, another negatively charged phospholipid, had no effect. Unlike the phospholipids used previously, however, phosphatidic acid has no polar head group and no phosphodiester bond. We concluded that the amebic receptor that recognized phosphatidylserine was charge specific and required the presence of a polar head group. In the context of the study of Bailey et al. (2), however, the receptor is not likely to be a stereospecific phosphatidylserine receptor like that identified in macrophages (13). Scavenger receptors that mediate the uptake of apoptotic cells in metazoans and that have the ability to bind phosphatidylserine are widely conserved (e.g., Ced-1 in Caenorhabditis elegans, Croquemort in Drosophila melanogaster, and CD36 in mammals), suggesting that the amebic phagocytosis receptor may be a scavenger receptor (32). However, a thorough search of the nearly complete E. histolytica genome databases (available through The Institute for Genomic Research and the Sanger Centre) identified no amebic homologues (unpublished results).
The nature of the amebic receptor(s) involved in the recognition of apoptotic cells remains an interesting and unresolved issue that we are pursuing. Multiple phagocyte receptors involved in the recognition of apoptotic cells have been described, including a specific phosphatidylserine receptor, lectins, integrins, and scavenger receptors (reviewed in reference 32). Indeed, the complexity and redundancy of ligands and receptors by which apoptotic cells are cleared in metazoans suggest that multiple amebic receptors and corresponding host cell ligands are likely.
The mechanism by which E. histolytica cell killing triggers the exposure of host cell phosphatidylserine is also an area of current investigation. Since the caspase 3 inhibitor Ac-DEVD-CHO at a concentration that blocks DNA fragmentation in cells exposed to E. histolytica did not reduce amebic phagocytosis, ligand exposure during amebic cell killing likely occurs upstream or independently of caspase 3 activation. Phosphatidylserine exposure on dying lymphocytes occurs via both caspase 3-dependent activation of protein kinase C-δ and Ca2+-dependent activation of a membrane phospholipid scramblase (3, 15, 22, 33, 37). Furthermore, the inhibition of caspase 3 reduces the quantity of phosphatidylserine exposed on dying lymphocytes but not the number of cells exposing phosphatidylserine (15). This and prior studies demonstrating a critical role for host cell Ca2+ fluxes early in amebic cell killing suggest a potential mechanism for phosphatidylserine exposure during amebic killing of host cells (28, 30).
This study is important because it begins to define a model of sequential E. histolytica adherence, apoptotic cell killing, and clearance. While a study with cinemicroscopy suggested that amebae kill host cells prior to phagocytosis (as determined by trypan blue uptake), a related study indicated that amebae ingest viable host cells (unstained by trypan blue) when GalNAc lectin-mediated cytotoxicity is blocked (26, 27). In contrast to these results, our study demonstrated a requirement for host cell apoptosis prior to ingestion by amebae, suggesting that the surface changes characteristic of apoptotic cells (e.g., phosphatidylserine exposure) trigger amebic phagocytosis. The data indicated that E. histolytica cytotoxicity and phagocytosis are not independent events, as previously concluded (27). Rather, they are stages in a sequence of events necessary for amebae to cause invasive disease.
Future in vivo studies testing this model may provide insight into why amebic cell killing and phagocytosis are critical to the ability of amebae to cause disease. Although brisk neutrophilic infiltration characterizes early amebic invasion, human autopsy studies have revealed very little reactive inflammation surrounding chronic amebic liver abscesses, and a similar paucity of inflammation has been noted at the base of well-formed colonic ulcers (1, 8, 19, 36). We hypothesize that the rapid clearance of apoptotically killed cells by amebae (and perhaps also by tissue macrophages) limits the leakage of toxic intracellular contents of killed cells. This effect would be consistent with the paucity of inflammatory infiltration surrounding well-established amebic liver and colonic lesions despite extraordinary tissue destruction and with the ability of E. histolytica to cause prolonged and/or progressive infection.
Acknowledgments
This work was supported by National Institutes of Health grant AI-26649 to W.A.P. and a Howard Hughes postdoctoral fellowship for physicians to C.D.H. W.A.P. is a Burroughs Wellcome Fund Scholar in Molecular Parasitology.
Editor: J. T. Barbieri
REFERENCES
- 1.Aikat, B. K., S. R. Bhusnurmath, A. K. Pal, P. N. Chhuttani, and D. V. Datta. 1979. The pathology and pathogenesis of fatal hepatic amoebiasis—a study based on 79 autopsy cases. Trans. R. Soc. Trop. Med. Hyg. 73:188-192. [DOI] [PubMed] [Google Scholar]
- 2.Bailey, G. B., D. B. Day, C. Nokkaew, and C. C. Harper. 1987. Stimulation by target cell membrane lipid of actin polymerization and phagocytosis by Entamoeba histolytica. Infect. Immun. 55:1848-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basse, F., J. G. Stout, P. J. Sims, and T. Wiedmer. 1996. Isolation of an erythrocyte membrane protein that mediates Ca2+-dependent transbilayer movement of phospholipid. J. Biol. Chem. 271:17205-17210. [DOI] [PubMed] [Google Scholar]
- 4.Berninghausen, O., and M. Leippe. 1997. Necrosis versus apoptosis as the mechanism of target cell death induced by Entamoeba histolytica. Infect. Immun. 65:3615-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyum, A. 1968. Isolation of mononuclear cells and granulocytess from human blood. Scand. J. Clin. Investig. 21(Suppl. 97):107-111. [PubMed] [Google Scholar]
- 6.Brandt, H., and R. Perez-Tamayo. 1970. The pathology of human amebiasis. Hum. Pathol. 1:351-385. [DOI] [PubMed] [Google Scholar]
- 7.Bratosin, D., J. Estaquier, F. Petit, D. Arnoult, B. Quatannens, J.-P. Tissier, C. Slomianny, C. Sartiaux, C. Alonso, J.-J. Huart, J. Montreuil, and J. C. Ameisen. 2001. Programmed cell death in mature erythrocytes: a model for investigating death effector pathways operating in the absence of mitochondria. Cell Death Differ. 8:1143-1156. [DOI] [PubMed] [Google Scholar]
- 8.Chadee, K., and E. Meerovitch. 1985. The pathology of experimentally induced cecal amebiasis in gerbils (Meriones unguiculatus). Liver changes and amebic liver abscess formation. Am. J. Pathol. 119:485-494. [PMC free article] [PubMed] [Google Scholar]
- 9.Cocco, R. E., and D. S. Ucker. 2001. Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure. Mol. Biol. Cell 12:919-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond, L. S., D. R. Harlow, and C. Cunnick. 1978. A new medium for axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72:431-432. [DOI] [PubMed] [Google Scholar]
- 11.Fadok, V. A., D. L. Bratton, and P. M. Henson. 2001. Phagocyte receptors for apoptotic cells: recognition, uptake, and consequences. J. Clin. Investig. 108:957-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fadok, V. A., A. de Cathelineau, D. L. Daleke, P. M. Henson, and D. L. Bratton. 2001. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 276:1071-1077. [DOI] [PubMed] [Google Scholar]
- 13.Fadok, V. A., D. L. Bratton, D. M. Rose, A. Pearson, R. A. B. Ezekewitz, and P. M. Henson. 2000. A receptor for phosphatidylserine specific clearance of apoptotic cells. Nature 405:85-90. [DOI] [PubMed] [Google Scholar]
- 14.Fadok, V. A., D. R. Voelker, P. A. Campbell, J. J. Cohen, D. L. Bratton, and P. M. Henson. 1992. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148:2207-2216. [PubMed] [Google Scholar]
- 15.Frasch, S. C., P. M. Henson, J. M. Kailey, D. A. Richter, M. S. Janes, V. A. Fadok, and D. L. Bratton. 2000. Regulation of phospholipid scramblase activity during apoptosis and cell activation by protein kinase C-delta. J. Biol. Chem. 275:23065-23073. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Ruiz, A., R. Haque, A. Aguirre, G. Castanon, A. Hall, F. Guhl, G. Ruiz-Palacios, M. A. Miles, and D. C. Warhurst. 1994. Value of microscopy in the diagnosis of dysentery associated with invasive Entamoeba histolytica. J. Clin. Pathol. 47:236-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess, K. L., G. F. Babcock, D. S. Askew, and J. M. Cook-Mills. 1997. A novel flow cytometric method for quantifying phagocytosis of apoptotic cells. Cytometry 27:145-152. [PMC free article] [PubMed] [Google Scholar]
- 18.Huston, C. D., E. R. Houpt, B. J. Mann, C. S. Hahn, and W. A. Petri. 2000. Caspase 3-dependent killing of host cells by the parasite Entamoeba histolytica. Cell. Microbiol. 2:617-625. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez, F. 1981. Pathology of amebiasis. Bull. N. Y. Acad. Med. 57:217-223. [PMC free article] [PubMed] [Google Scholar]
- 20.Maltz, G., and C. M. Knauer. 1991. Amebic liver abscess: a 15-year experience. Am. J. Gastroenterol. 86:704-710. [PubMed] [Google Scholar]
- 21.Martinez-Palomo, A., A. Gonzalez-Robles, B. Chavez, E. Orozco, S. Fernandez-Castelo, and A. Cervantes. 1985. Structural bases of the cytolytic mechanisms of Entamoeba histolytica. J. Protozool. 32:166-175. [DOI] [PubMed] [Google Scholar]
- 22.McEvoy, L., P. Williamson, and R. A. Schlegel. 1986. Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc. Natl. Acad. Sci. USA 83:3311-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orozco, E., G. Guarneros, and A. Martinez-Palomo. 1983. Entamoeba histolytica: phagocytosis as a virulence factor. J. Exp. Med. 158:1511-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orozco, E., M. A. Rodriguez, and F. C. Hernandez. 1988. The role of phagocytosis in the pathogenic mechanism of Entamoeba histolytica, p. 326-338. In J. I. Ravdin (ed.), Amebiasis: human infection by Entamoeba histolytica. John Wiley & Sons, Inc., New York, N.Y.
- 25.Petri, W. A., Jr., R. D. Smith, P. H. Schlesinger, C. F. Murphy, and J. I. Ravdin. 1987. Isolation of the galactose binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J. Clin. Investig. 80:1238-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravdin, J. I., B. Y. Croft, and R. L. Guerrant. 1980. Cytopathogenic mechanisms of Entamoeba histolytica. J. Exp. Med. 152:377-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravdin, J. I., and R. L. Guerrant. 1981. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J. Clin. Investig. 68:1305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravdin, J. I., C. F. Murphy, R. L. Guerrant, and S. A. Long-Krug. 1985. Effect of antagonists of calcium and phospholipase A on the cytopathogenicity of Entamoeba histolytica. J. Infect. Dis. 152:542-549. [DOI] [PubMed] [Google Scholar]
- 29.Ravdin, J. I., P. H. Schlesinger, C. F. Murphy, I. Y. Gluzman, and D. J. Krogstad. 1986. Acid intracellular vesicles and the cytolysis of mammalian target cells by Entamoeba histolytica trophozoites. J. Protozool. 33:478-486. [DOI] [PubMed] [Google Scholar]
- 30.Ravdin, J. I., N. Sperelakis, and R. L. Guerrant. 1982. Effect of ion channel inhibitors on the cytopathogenicity of Entamoeba histolytica. J. Infect. Dis. 146:335-340. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez, M. A., and E. Orozco. 1986. Isolation and characterization of phagocytosis- and virulence-deficient mutants of Entamoeba histolytica. J. Infect. Dis. 154:27-32. [DOI] [PubMed] [Google Scholar]
- 32.Savill, J., and V. Fadok. 2000. Corpse clearance defines the meaning of cell death. Nature 407:784-788. [DOI] [PubMed] [Google Scholar]
- 33.Schlegel, R. A., M. K. Callahan, and P. Williamson. 2000. The central role of phosphatidylserine in the phagocytosis of apoptotic thymocytes. Ann. N. Y. Acad. Sci. 926:217-225. [DOI] [PubMed] [Google Scholar]
- 34.Seydel, K. B., and S. L. Stanley, Jr. 1998. Entamoeba histolytica induces host cell death in amebic liver abscess by a non-Fas-dependent, non-tumor necrosis factor alpha-dependent pathway of apoptosis. Infect. Immun. 66:2980-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trissl, D., A. Martinez-Palomo, M. de la Torre, R. de la Hoz, and E. P. de Suarez. 1978. Surface properties of Entamoeba: increased rates of human erythrocyte phagocytosis in pathogenic strains. J. Exp. Med. 148:1137-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsutsumi, V., and A. Martinez-Palomo. 1988. Inflammatory reaction in experimental hepatic amebiasis: an ultrastructural study. Am. J. Pathol. 130:112-119. [PMC free article] [PubMed] [Google Scholar]
- 37.Williamson, P., A. Christie, T. Kohlin, R. A. Schlegel, P. Comfurius, M. Harmsma, R. F. A. Zwaal, and E. M. Bevers. 2001. Phospholipid scramblase activation pathways in lymphocytes. Biochemistry 40:8065-8072. [DOI] [PubMed] [Google Scholar]
- 38.Yan, L., and S. L. Stanley, Jr. 2001. Blockade of caspases inhibits amebic liver abscess formation in a mouse model of disease. Infect. Immun. 69:7911-7914. [DOI] [PMC free article] [PubMed] [Google Scholar]