Abstract
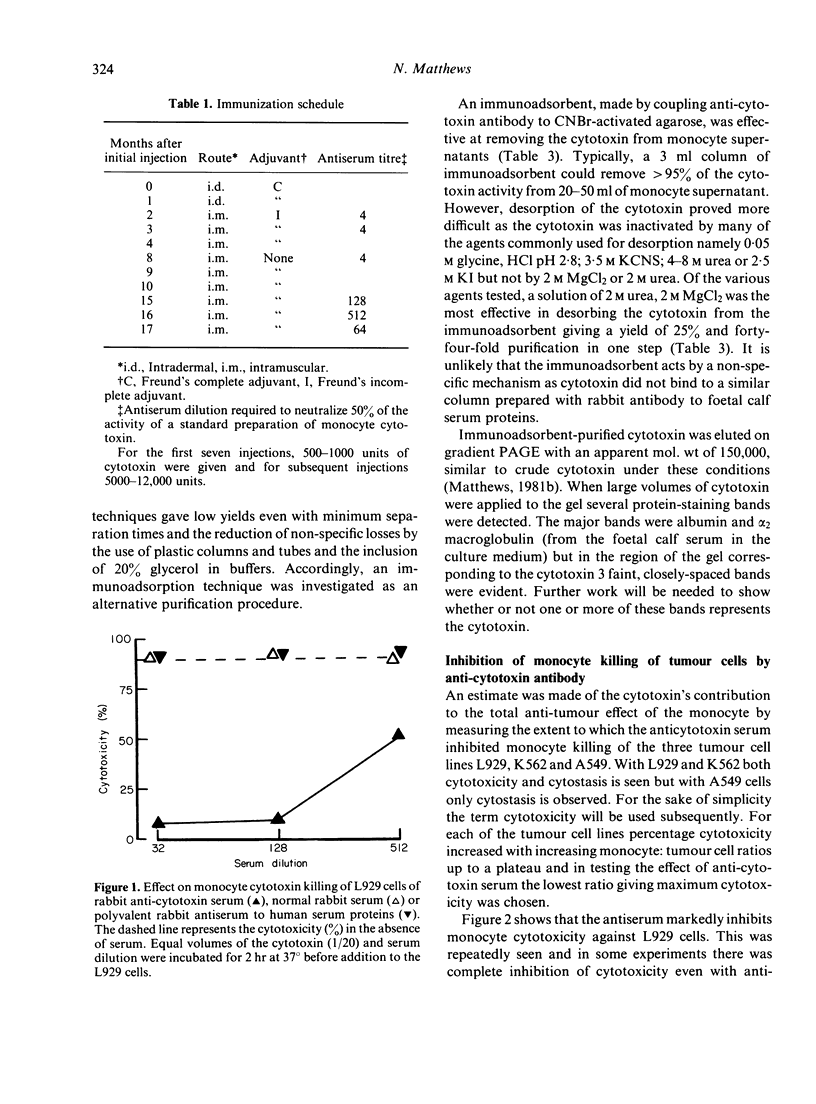
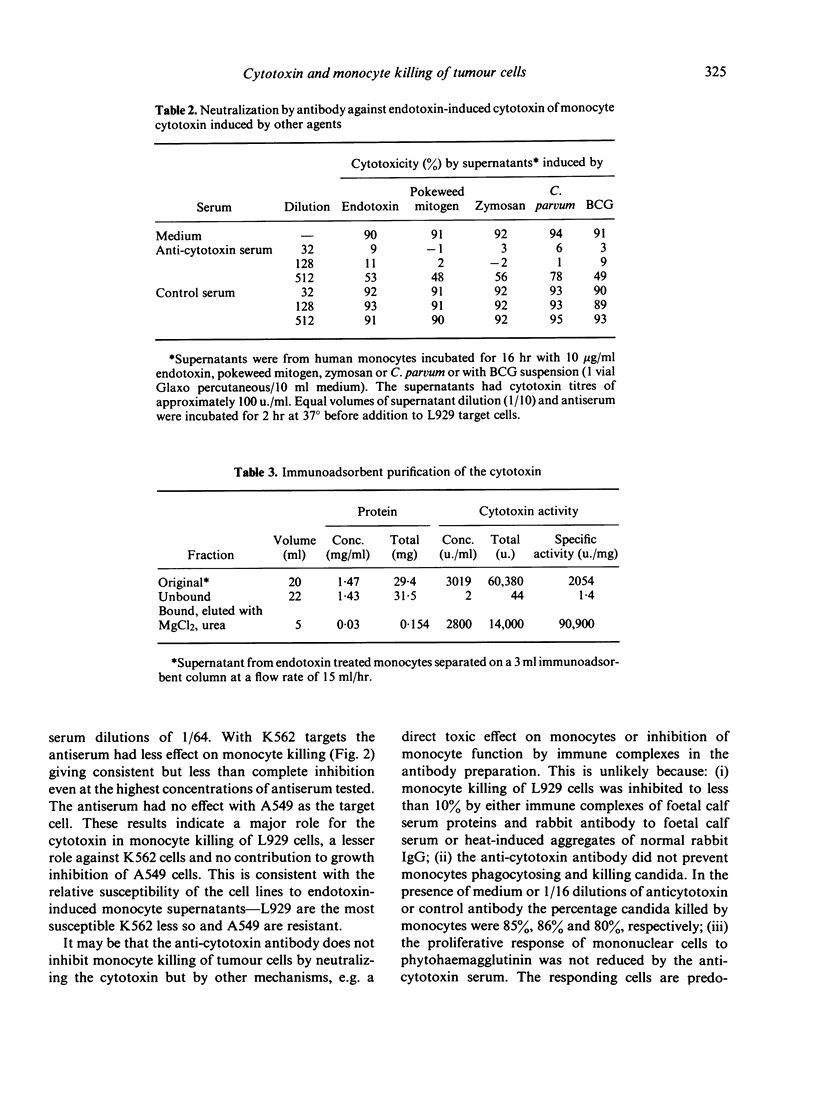
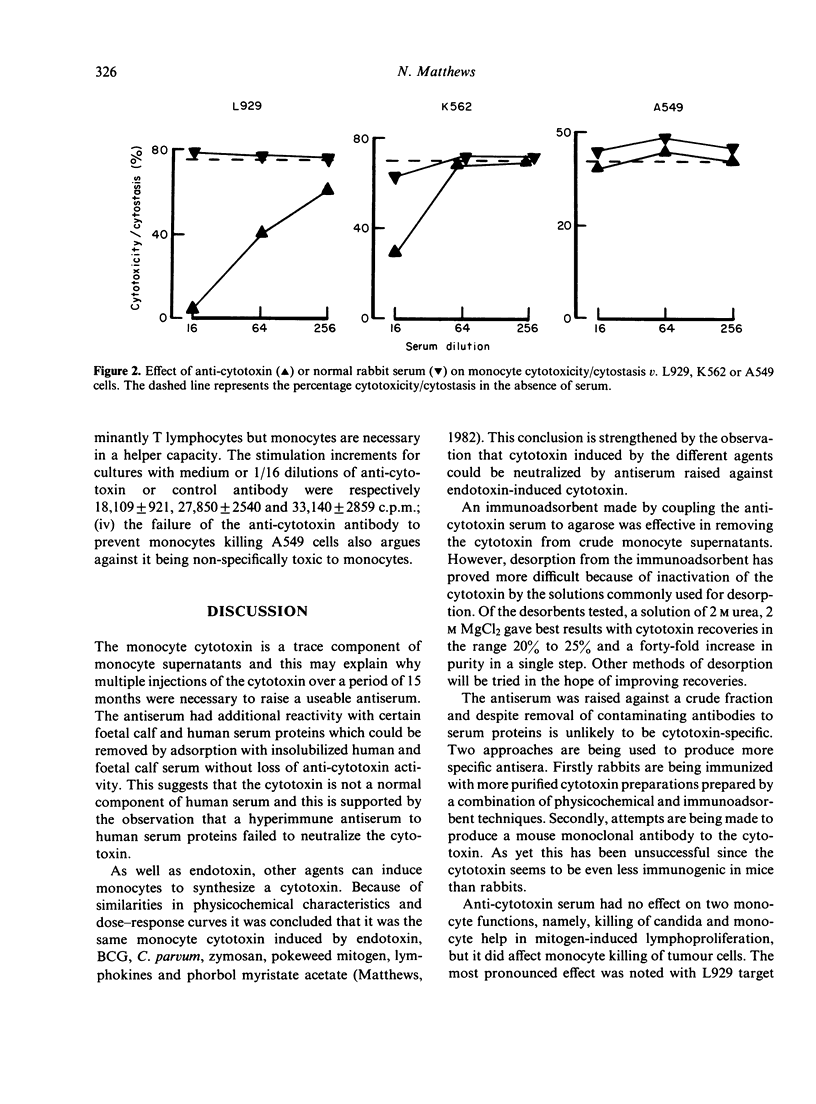
Human monocytes can kill or inhibit the growth of certain tumour cell lines. Amongst the possible mediators is a cytotoxin synthesized in readily detectable amounts by endotoxin-stimulated monocytes. A neutralizing antiserum to the cytotoxin has been used to assess the cytotoxin's contribution to monocyte killing of tumour cells. The antiserum was tested for possible inhibition of monocyte killing of three tumour cell lines--L929, K562 and A549. Inhibition was complete with L929, partial with K562 and insignificant with A549. Thus the contribution of the cytotoxin to monocyte killing of tumour cells depends upon the tumour line under test. Antibody against endotoxin-induced cytotoxin also neutralized cytotoxin induced in monocytes by other agents including BCG, Corynebacterium parvum, pokeweed mitogen and zymosan. Cytotoxin could be quantitatively removed from monocyte supernatants by Sepharose-bound, anticytotoxin antibody. Recovery of the cytotoxin from the immunoadsorbent was difficult because of its lability in the solutions commonly used for desorption. The best recovery has been achieved with 2 M urea, 2 M MgCl2 giving a 25% yield and a forty-fold increase in purity in a single step.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Kao K. J., Farb R., Pizzo S. V. Effector mechanisms of cytolytically activated macrophages. II. Secretion of a cytolytic factor by activated macrophages and its relationship to secreted neutral proteases. J Immunol. 1980 Jan;124(1):293–300. [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Ferluga J., Schorlemmer H. U., Baptista L. C., Allison A. C. Production of the complement cleavage product, C3a, by activated macrophages and its tumorolytic effects. Clin Exp Immunol. 1978 Mar;31(3):512–517. [PMC free article] [PubMed] [Google Scholar]
- Johnson W. J., Whisnant C. C., Adams D. O. The binding of BCG-activated macrophages to tumor targets stimulates secretion of cytolytic factor. J Immunol. 1981 Nov;127(5):1787–1792. [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Furth R. Kinetics of phagocytosis and intracellular killing of Candida albicans by human granulocytes and monocytes. Infect Immun. 1977 Aug;17(2):313–318. doi: 10.1128/iai.17.2.313-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N. Production of an anti-tumour cytotoxin by human monocytes. Immunology. 1981 Sep;44(1):135–142. [PMC free article] [PubMed] [Google Scholar]
- Matthews N. Production of an anti-tumour cytotoxin by human monocytes: comparison of endotoxin, interferons and other agents as inducers. Br J Cancer. 1982 Apr;45(4):615–617. doi: 10.1038/bjc.1982.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N. Tumour-necrosis factor from the rabbit. II. Production by monocytes. Br J Cancer. 1978 Aug;38(2):310–315. doi: 10.1038/bjc.1978.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N. Tumour-necrosis factor from the rabbit. V. Synthesis in vitro by mononuclear phagocytes from various tissues of normal and BCG-injected rabbits. Br J Cancer. 1981 Sep;44(3):418–424. doi: 10.1038/bjc.1981.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Falk W., Meltzer M. S. Inhibition of nonspecific tumoricidal activity by activated macrophages with antiserum against a soluble cytotoxic factor. Infect Immun. 1981 Jul;33(1):156–164. doi: 10.1128/iai.33.1.156-164.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Moore R. N., Mergenhagen S. E. Macrophages as a source of tumoricidal activity (tumor-necrotizing factor). Infect Immun. 1980 Nov;30(2):523–530. doi: 10.1128/iai.30.2.523-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]


