Abstract
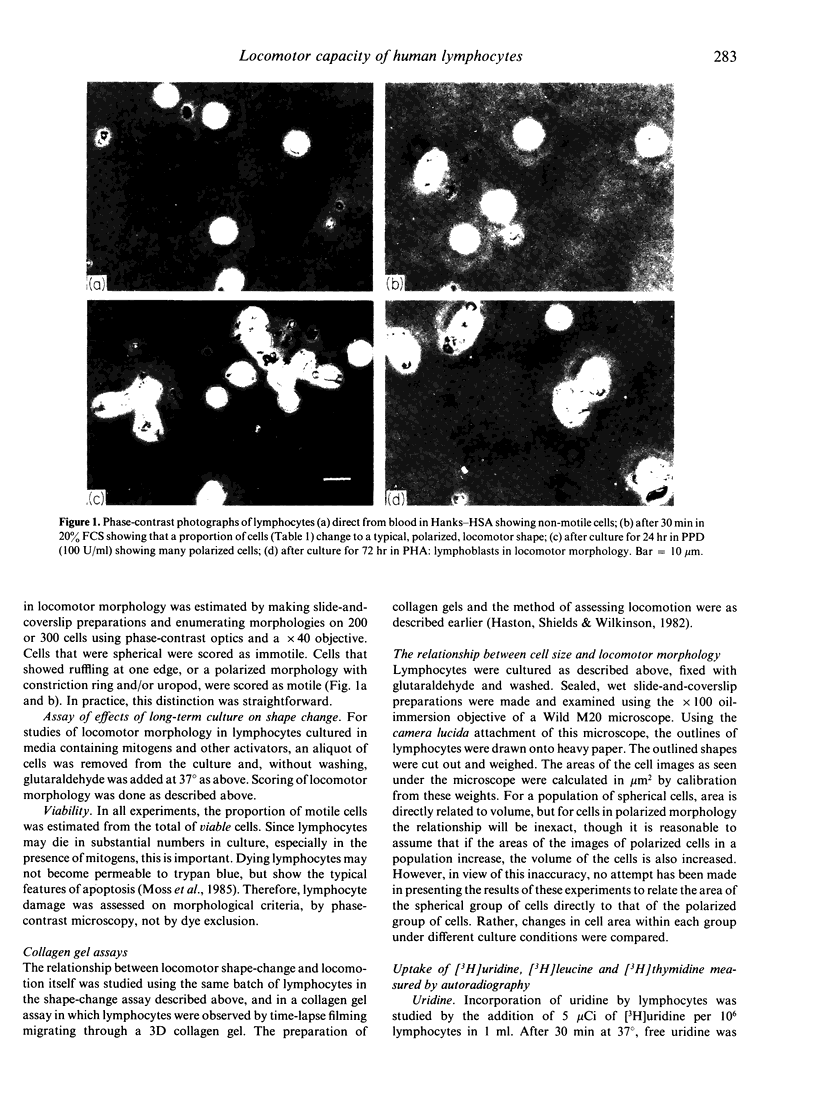
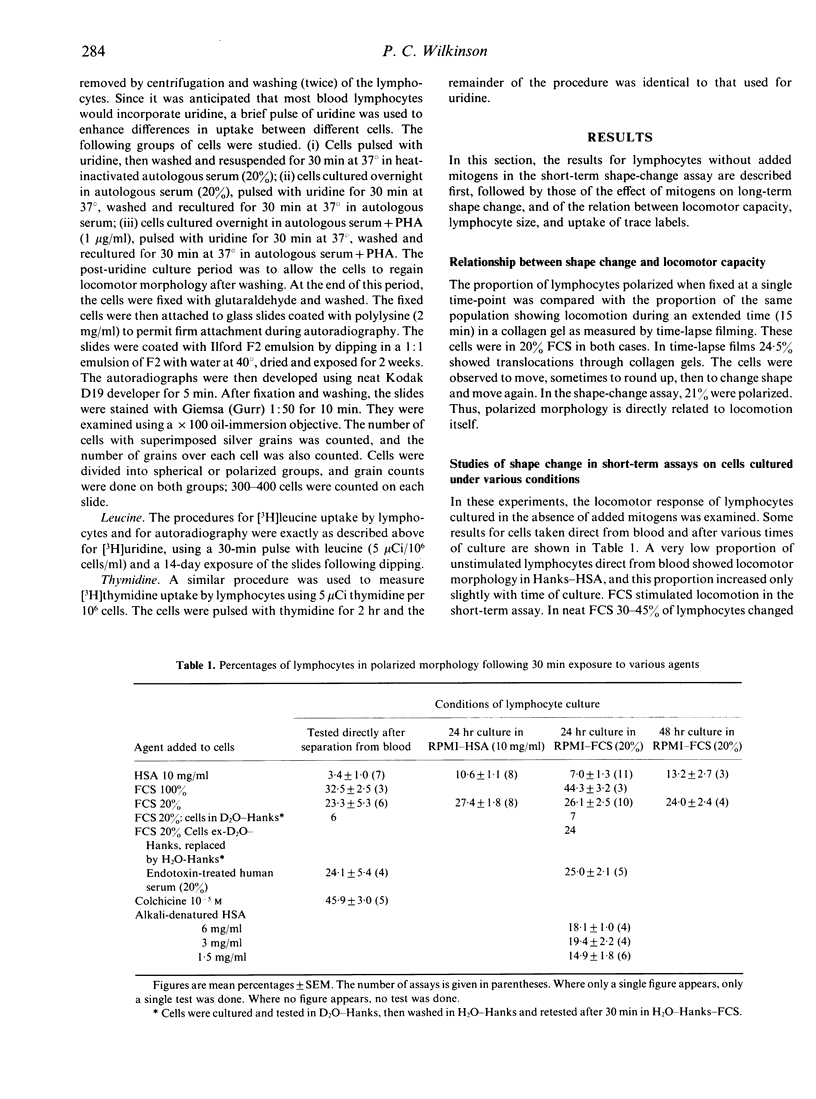
The locomotor capacity of human blood lymphocytes taken directly from blood or cultured in various ways was measured by the change from a spherical to a polarized shape which occurs within minutes of adding locomotor stimulants. A minority of lymphocytes, either direct from blood or after culture in human serum albumin or fetal calf serum for up to 72 hr, responded rapidly to such stimulants, but most lymphocytes failed to show any shape change. Colchicine induced the highest proportion of polarized cells, though still below 50%, and deuterium oxide, which stabilizes microtubule assembly, inhibited shape-change, suggesting that microtubules have a regulatory function in the expression of lymphocyte locomotion. However culture in the presence of mitogens, namely, phytohaemagglutinin (PHA), PPD, mixed lymphocyte culture, or anti-T3 (OKT3 greater than or equal to 25 pg/ml), caused a majority of lymphocytes to change shape slowly over a period of hours. In the presence of mitogens, a high proportion of cells was already polarized after 24 hr in culture without addition of further locomotor stimulants. It was concluded that locomotor capacity in lymphocytes is dependent on growth and synthesis for the following reasons. (i) There was a direct relationship between size and locomotor morphology in PHA-cultured lymphocytes. Those lymphocytes that increased in size also became polarized. (ii) Autoradiography showed that the polarized cells were more active in [3H]uridine and [3H]leucine uptake than the spherical cells. This relationship was obvious in PHA-cultured cells but was also evident even in cells direct from blood. The increase in locomotor morphology preceded detectable DNA synthesis ([3H]thymidine uptake). (iii) Increase in locomotor capacity in culture was inhibited by cycloheximide but not by mitomycin c. These findings suggest that those cells most active in RNA and protein synthesis are also the most actively motile, and that, during culture with mitogens, locomotor capacity increases as G1 phase progresses and prior to the commencement of DNA synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottazzi B., Introna M., Allavena P., Villa A., Mantovani A. In vitro migration of human large granular lymphocytes. J Immunol. 1985 Apr;134(4):2316–2321. [PubMed] [Google Scholar]
- Center D. M., Cruikshank W. Modulation of lymphocyte migration by human lymphokines. I. Identification and characterization of chemoattractant activity for lymphocytes from mitogen-stimulated mononuclear cells. J Immunol. 1982 Jun;128(6):2563–2568. [PubMed] [Google Scholar]
- Cianciolo G. J., Snyderman R. Monocyte responsiveness to chemotactic stimuli is a property of a subpopulation of cells that can respond to multiple chemoattractants. J Clin Invest. 1981 Jan;67(1):60–68. doi: 10.1172/JCI110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Naggar A. K., Van Epps D. E., Williams R. C., Jr Human-B and T-lymphocyte locomotion in response to casein, C5a, and f-met-leu-phe. Cell Immunol. 1980 Dec;56(2):365–373. doi: 10.1016/0008-8749(80)90112-4. [DOI] [PubMed] [Google Scholar]
- El-Naggar A., Van Epps D. E., Williams R. C., Jr A human lymphocyte chemotactic factor produced by the mixed lymphocyte reaction. J Lab Clin Med. 1982 Oct;100(4):558–565. [PubMed] [Google Scholar]
- El-Naggar A., Van Epps D. E., Williams R. C., Jr Effect of culturing on the human lymphocyte locomotion response to casein, C5a, and fMet-Leu-Phe. Cell Immunol. 1981 May 1;60(1):43–49. doi: 10.1016/0008-8749(81)90246-x. [DOI] [PubMed] [Google Scholar]
- Fontana J. A., Wright D. G., Schiffman E., Corcoran B. A., Deisseroth A. B. Development of chemotactic responsiveness in myeloid precursor cells: studies with a human leukemia cell line. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3664–3668. doi: 10.1073/pnas.77.6.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
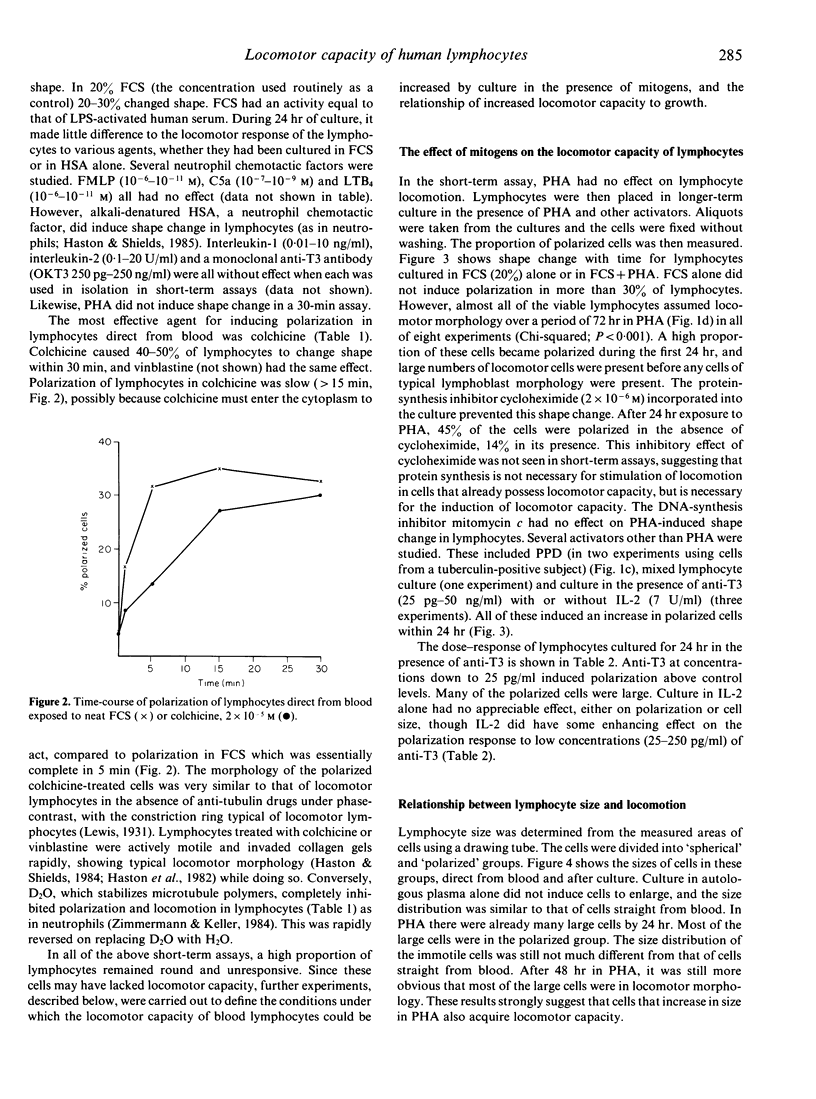
- Haston W. S., Shields J. M. Contraction waves in lymphocyte locomotion. J Cell Sci. 1984 Jun;68:227–241. doi: 10.1242/jcs.68.1.227. [DOI] [PubMed] [Google Scholar]
- Haston W. S., Shields J. M. Neutrophil leucocyte chemotaxis: a simplified assay for measuring polarizing responses to chemotactic factors. J Immunol Methods. 1985 Aug 2;81(2):229–237. doi: 10.1016/0022-1759(85)90208-x. [DOI] [PubMed] [Google Scholar]
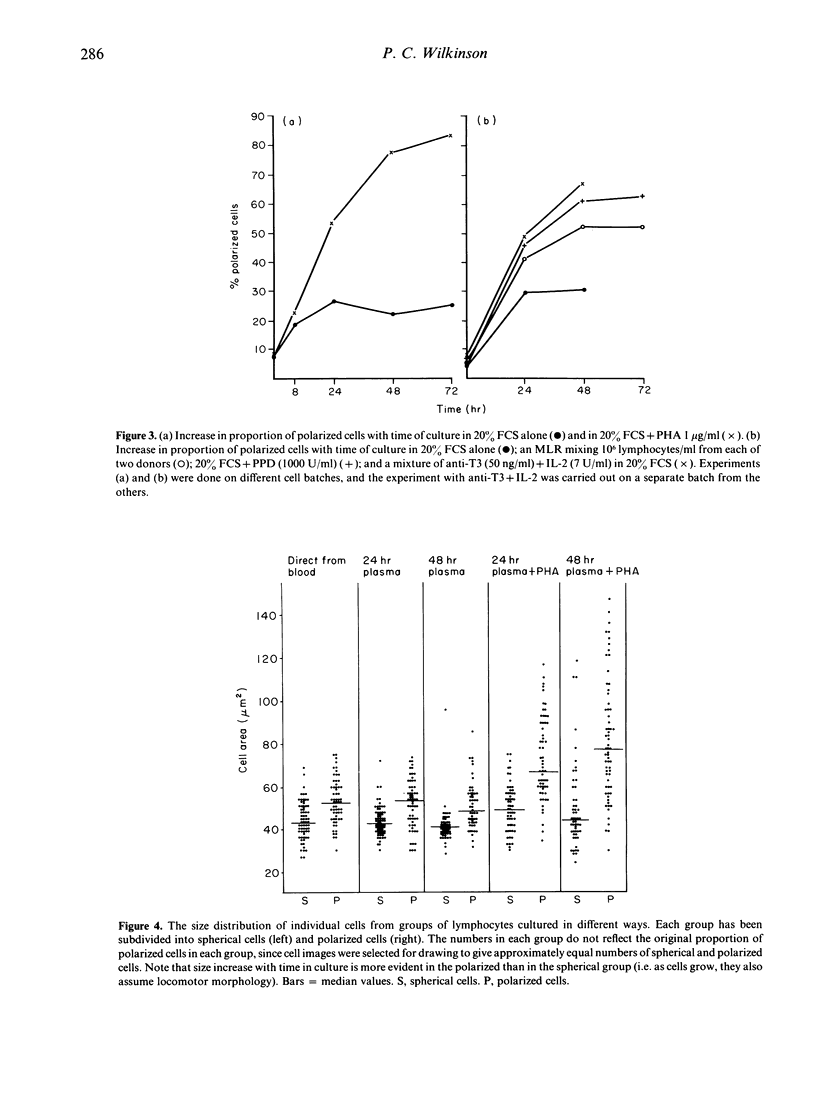
- Haston W. S., Shields J. M., Wilkinson P. C. Lymphocyte locomotion and attachment on two-dimensional surfaces and in three-dimensional matrices. J Cell Biol. 1982 Mar;92(3):747–752. doi: 10.1083/jcb.92.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoué S., Sato H. Cell motility by labile association of molecules. The nature of mitotic spindle fibers and their role in chromosome movement. J Gen Physiol. 1967 Jul;50(6 Suppl):259–292. [PMC free article] [PubMed] [Google Scholar]
- Keller H. U., Naef A., Zimmermann A. Effects of colchicine, vinblastine and nocodazole on polarity, motility, chemotaxis and cAMP levels of human polymorphonuclear leukocytes. Exp Cell Res. 1984 Jul;153(1):173–185. doi: 10.1016/0014-4827(84)90459-2. [DOI] [PubMed] [Google Scholar]
- Keller H. U., Zimmermann A., Cottier H. Crawling-like movements, adhesion to solid substrata and chemokinesis of neutrophil granulocytes. J Cell Sci. 1983 Nov;64:89–106. doi: 10.1242/jcs.64.1.89. [DOI] [PubMed] [Google Scholar]
- Moss D. J., Bishop C. J., Burrows S. R., Ryan J. M. T lymphocytes in infectious mononucleosis. I. T cell death in vitro. Clin Exp Immunol. 1985 Apr;60(1):61–69. [PMC free article] [PubMed] [Google Scholar]
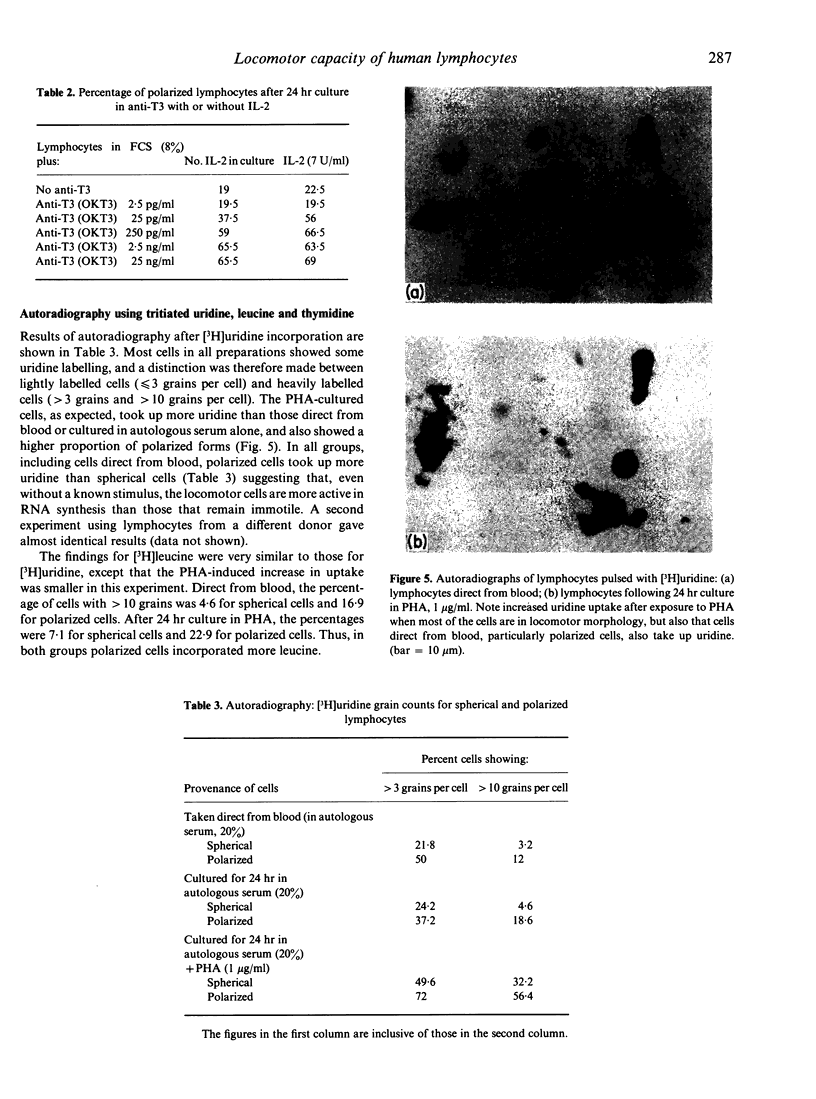
- Niedel J., Kahane I., Lachman L., Cuatrecasas P. A subpopulation of cultured human promyelocytic leukemia cells (HL-60) displays the formyl peptide chemotactic receptor. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1000–1004. doi: 10.1073/pnas.77.2.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill G. J., Parrott D. M. Locomotion of human lymphoid cells. I. Effect of culture and con A on T and non-T lymphocytes. Cell Immunol. 1977 Oct;33(2):257–267. doi: 10.1016/0008-8749(77)90156-3. [DOI] [PubMed] [Google Scholar]
- Parrott D. M., Wilkinson P. C. Lymphocyte locomotion and migration. Prog Allergy. 1981;28:193–284. [PubMed] [Google Scholar]
- Russell R. J., Wilkinson P. C., Sless F., Parrott D. M. Chemotaxis of lymphoblasts. Nature. 1975 Aug 21;256(5519):646–648. doi: 10.1038/256646a0. [DOI] [PubMed] [Google Scholar]
- Saklatvala J., Pilsworth L. M., Sarsfield S. J., Gavrilovic J., Heath J. K. Pig catabolin is a form of interleukin 1. Cartilage and bone resorb, fibroblasts make prostaglandin and collagenase, and thymocyte proliferation is augmented in response to one protein. Biochem J. 1984 Dec 1;224(2):461–466. doi: 10.1042/bj2240461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. The modulation of spontaneous and anti-Ig-stimulated motility of lymphocytes by cyclic nucleotides and adrenergic and cholinergic agents. J Immunol. 1975 Feb;114(2 Pt 2):802–808. [PubMed] [Google Scholar]
- Shields J. M., Haston W. S. Behaviour of neutrophil leucocytes in uniform concentrations of chemotactic factors: contraction waves, cell polarity and persistence. J Cell Sci. 1985 Mar;74:75–93. doi: 10.1242/jcs.74.1.75. [DOI] [PubMed] [Google Scholar]
- Van Epps D. E., Potter J. W., Durant D. A. Production of a human T lymphocyte chemotactic factor by T cell subpopulations. J Immunol. 1983 Jun;130(6):2727–2731. [PubMed] [Google Scholar]
- Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger L., Otteskog P., Sundqvist K. G. Anchorage and lymphocyte function. Acquisition of spontaneous motile behaviour by human blood lymphocytes and its modulation by concanavalin A. Immunology. 1985 Nov;56(3):425–434. [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Unanue E. R., Goralnick S. J., Schreiner G. F. Chemotaxis of rat lymphocytes. J Immunol. 1977 Aug;119(2):416–421. [PubMed] [Google Scholar]
- Wilkinson P. C., Bradley G. R. Chemotactic and enzyme-releasing activity of amphipathic proteins for neutrophils. A possible role for protease in chemotaxis on substratum-bound protein gradients. Immunology. 1981 Apr;42(4):637–648. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C., Parrott D. M., Russell R. J., Sless F. Antigen-induced locomotor responses in lymphocytes. J Exp Med. 1977 May 1;145(5):1158–1168. doi: 10.1084/jem.145.5.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann B., Damerau B., Vogt W. Purification and partial amino acid sequence of classical anaphylatoxin from pig serum: identification with Des-Arg-C5a. Hoppe Seylers Z Physiol Chem. 1980;361(6):915–924. doi: 10.1515/bchm2.1980.361.1.915. [DOI] [PubMed] [Google Scholar]
- van Epps D. E., Chenoweth D. E. Analysis of the binding of fluorescent C5a and C3a to human peripheral blood leukocytes. J Immunol. 1984 Jun;132(6):2862–2867. [PubMed] [Google Scholar]