Abstract
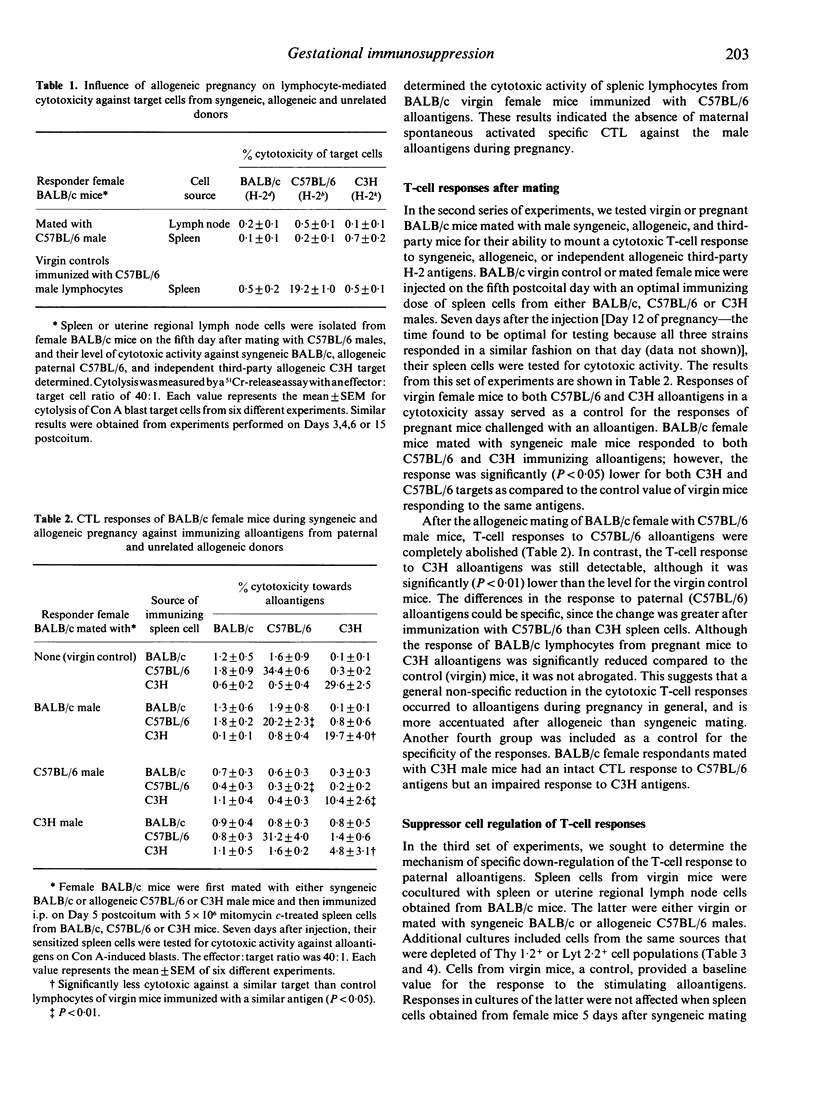
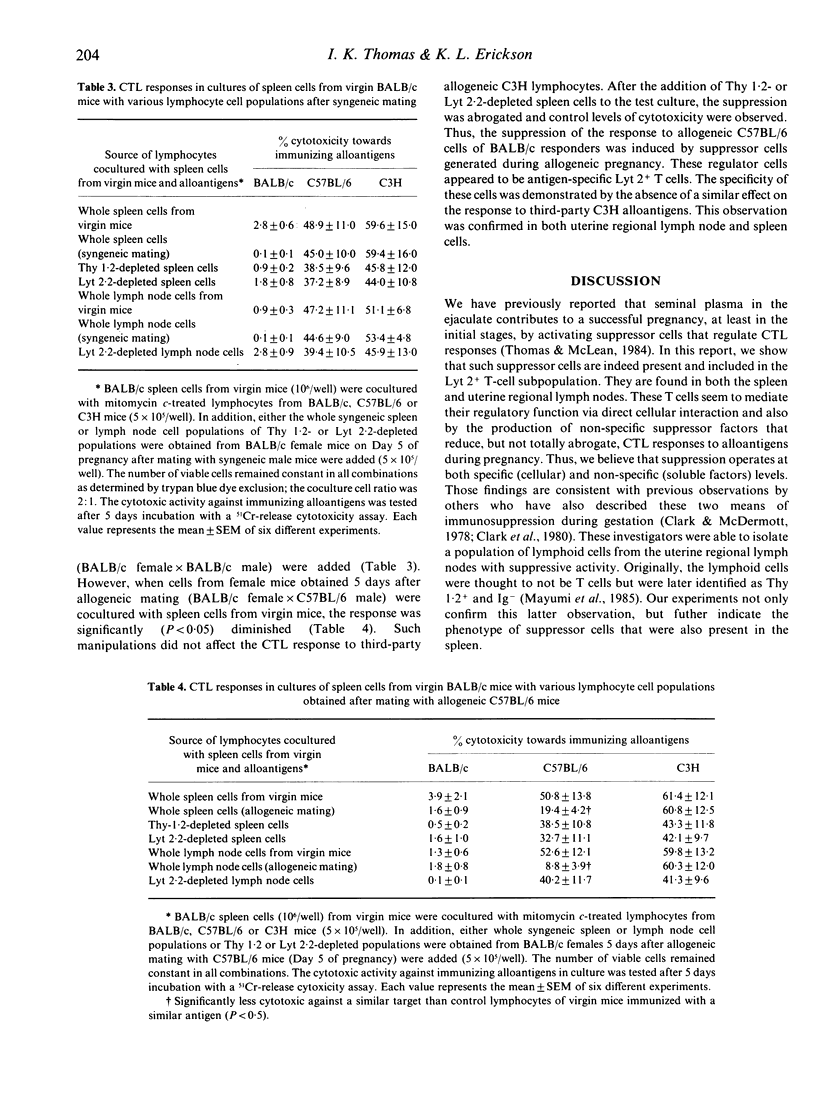
Female BALB/c mice were tested during the first week of pregnancy for their lymphocyte-mediated cytotoxic response to paternal alloantigens. Spleen or uterine regional lymph node cells were not spontaneously cytotoxic against concanavalin A-activated paternal target lymphocytes. Female mice immunized i.p. with paternal H-2-matched or third-party allogeneic cells on the fifth day and tested on the 12th day of pregnancy demonstrated total suppression of cell-mediated cytotoxicity to paternal alloantigens and partial suppression to third-party alloantigens. A generalized non-specific immunosuppression to alloantigens seems to be associated with pregnancy, which may indicate that soluble factors were involved in mediating the suppressive effect. Cocultures of spleen cells from virgin mice and the whole population of spleen or regional lymph node cells from allogeneic pregnant female mice demonstrated specifically suppressed responses to alloantigens. Similar cocultures with Thy 1.2- and Lyt 2.2-depleted populations restored the cytotoxicity levels of activated spleen cells. We conclude that antigen-specific Lyt 2+ T cells were activated during pregnancy to regulate the female T-cell response to paternal alloantigens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boehmer H., Sprent J., Nabholz M. Tolerance to histocompatibility determinants in tetraparental bone marrow chimeras. J Exp Med. 1975 Feb 1;141(2):322–334. doi: 10.1084/jem.141.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C. G., Brent L., Kilshaw P. J., New R. R., Pinto M. Specific unresponsiveness to skin allografts in mice. IV. Immunological reactivity of mice treated with liver extracts, Bordetella pertussis, and antilymphocyte serum. Transplantation. 1975 Feb;19(2):134–144. [PubMed] [Google Scholar]
- Ceppellini R., Bonnard G. D., Coppo F., Miggiano V. C., Pospisil M., Curtoni E. S., Pellegrino M. Transplantation antigens: introductory symposium. Mixed leukocyte cultures and HL-A antigens. I. Reactivity of young fetuses, newborns and mothers at delivery. Transplant Proc. 1971 Mar;3(1):58–63. [PubMed] [Google Scholar]
- Chaouat G., Voisin G. A. Regulatory T cell subpopulations in pregnancy. I. Evidence for suppressive activity of the early phase of MLR. J Immunol. 1979 Apr;122(4):1383–1388. [PubMed] [Google Scholar]
- Clark D. A., McDermott M. R. Active suppression of host-vs-graft reaction in pregnant mice. III. Developmental kinetics, properties, and mechanism of induction of suppressor cells during first pregnancy. J Immunol. 1981 Oct;127(4):1267–1273. [PubMed] [Google Scholar]
- Clark D. A., McDermott M. R. Impairment of host vs graft reaction in pregnant mice. I. Suppression of cytotoxic T cell generation in lymph nodes draining the uterus. J Immunol. 1978 Oct;121(4):1389–1393. [PubMed] [Google Scholar]
- Clark D. A., McDermott M. R., Szewczuk M. R. Impairment of host-versus-graft reaction in pregnant mice. II. Selective suppression of cytotoxic T-cell generation correlates with soluble suppressor activity and with successful allogeneic pregnancy. Cell Immunol. 1980 Jun;52(1):106–118. [PubMed] [Google Scholar]
- Engleman E. G., McMichael A. J., Batey M. E., McDevitt H. O. A suppressor T cell of the mixed lymphocyte reaction in man specific for the stimulating alloantigen. Evidence that identity at HLA-D between suppressor and responder is required for suppression. J Exp Med. 1978 Jan 1;147(1):137–146. doi: 10.1084/jem.147.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayumi T., Bitoh S., Anan S., Hama T., Fujimoto S., Yamamoto H. Suppressor T lymphocyte induction by a factor released from cultured blastocysts. J Immunol. 1985 Jan;134(1):404–409. [PubMed] [Google Scholar]
- Rieger M., Günther J., Kristofová H., Hilgert I. Evidence of suppresssor cell-mechanism of allograft tolerance induced by spleen extract and hydrocortisone. Folia Biol (Praha) 1978;24(3):145–161. [PubMed] [Google Scholar]
- Rocklin R. E., Kitzmiller J. L., Carpenter C. B., Garovoy M. R., David J. R. Maternal-fetal relation. Absence of an immunologic blocking factor from the serum of women with chronic abortions. N Engl J Med. 1976 Nov 25;295(22):1209–1213. doi: 10.1056/NEJM197611252952201. [DOI] [PubMed] [Google Scholar]
- Simpson M. A., Gozzo J. J. Isolation of suppressor cells from immunosuppressed and antigen-challenged mice. Transplant Proc. 1979 Jun;11(2):1452–1453. [PubMed] [Google Scholar]
- Thomas I. K., Erickson K. L. Lipid modulation of mammary tumor cell cytolysis: direct influence of dietary fats on the effector component of cell-mediated cytotoxicity. J Natl Cancer Inst. 1985 Mar;74(3):675–680. [PubMed] [Google Scholar]
- Thomas I. K., Erickson K. L. Seminal plasma inhibits lymphocyte response to T-dependent and -independent antigens in vitro. Immunology. 1984 Aug;52(4):721–726. [PMC free article] [PubMed] [Google Scholar]
- Thomas I. K., Leeming G., Gibbs A. C., McLean J. M. A specific cytolytic T cell response induced by subcutaneous challenge with allogeneic rat epididymal spermatozoa. J Reprod Immunol. 1981 Jul;3(3):157–164. doi: 10.1016/0165-0378(81)90057-7. [DOI] [PubMed] [Google Scholar]
- Thomas I. K., McLean J. M. Seminal plasma abrogates the postcoital T cell response to spermatozoal histocompatibility antigens. Am J Reprod Immunol. 1984 Dec;6(4):185–189. doi: 10.1111/j.1600-0897.1984.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Vanderbeeken Y., Vlieghe M. P., Delespesse G., Duchateau J. Characterization of immunoregulatory T cells during pregnancy by monoclonal antibodies. Clin Exp Immunol. 1982 Apr;48(1):118–120. [PMC free article] [PubMed] [Google Scholar]
- Vanderbeeken Y., Vlieghe M. P., Duchateau J., Delespesse G. Suppressor T-lymphocytes in pregnancy. Am J Reprod Immunol. 1984 Jan-Feb;5(1):20–24. doi: 10.1111/j.1600-0897.1984.tb00282.x. [DOI] [PubMed] [Google Scholar]
- Wegmann T. G., Waters C. A., Drell D. W., Carlson G. A. Pregnant mice are not primed but can be primed to fetal alloantigens. Proc Natl Acad Sci U S A. 1979 May;76(5):2410–2414. doi: 10.1073/pnas.76.5.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]


