Abstract
Shiga toxin-producing enterohemorrhagic Escherichia coli is the major cause of acute renal failure in young children. The interaction of Shiga toxins 1 and 2 (Stx1 and Stx2) with endothelial cells is an important step in the renal coagulation and thrombosis observed in hemolytic uremic syndrome. Previous studies have shown that bacterial lipopolysaccharide and host cytokines slowly sensitize endothelial cells to Shiga toxins. In the present study, bacterial neutral sphingomyelinase (SMase) rapidly (1 h) sensitized human dermal microvascular endothelial cells (HDMEC) to the cytotoxic action of Stx2. Exposure of endothelial cells to neutral SMase (0.067 U/ml) caused a rapid increase of intracellular ceramide that persisted for hours. Closely following the change in ceramide level was an increase in the expression of globotriaosylceramide (Gb3), the receptor for Stx2. A rapid increase was also observed in the mRNA for ceramide:glucosyltransferase (CGT), the first of three glycosyltransferase enzymes of the Gb3 biosynthetic pathway. The product of CGT (glucosylceramide) was also increased. In contrast, mRNA for the third enzyme of the pathway, Gb3 synthase, was constitutively produced and was not influenced by SMase treatment of HDMEC. These results describe a rapid response mechanism by which extracellular neutral SMase derived from either bacteria or eukaryotic cells may signal endothelial cells to become sensitive to Shiga toxins.
Enterohemorrhagic Escherichia coli (EHEC) O157:H7-associated hemolytic uremic syndrome is largely due to the action of Shiga toxin 1 (Stx1) and Stx2 on specific cells and target organs (19, 23, 29). It is well documented that Stx1 and Stx2 directly affect human endothelial cells (20, 21, 33). The glycosphingolipid globotriaosylceramide (Gb3) is the receptor for Shiga toxins that must be expressed on the surfaces of toxin-sensitive cells (10, 12). In vitro, Gb3 may be induced by bacterial lipopolysaccharide (LPS) or host cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) (15-18, 21, 25, 34, 36). Previous studies have demonstrated that LPS, TNF-α, and IL-1β require approximately 24 to 48 h for maximal induction of Gb3 on endothelial cells and that LPS or TNF-α requires signal transduction via protein kinase C (18).
The present study describes an alternative agent, neutral sphingomyelinase (nSMase), that rapidly induces Stx sensitivity in human endothelial cells. Within the family of SMase enzymes, nSMases are derived from both prokaryotic and eukaryotic sources whereas acidic SMases (aSMases) are primarily found within eukaryotic organelles (13, 14, 22, 38). Neutral and acidic refer to the pH required for optimal enzymatic activity. Numerous biological effects in eukaryotic cells have been attributed to nSMase action, when resident SMase is activated either in the plasma membrane or in response to extracellular SMase (2, 4, 13, 38). In both cases, SMase hydrolyzes endogenous sphingomyelin in the plasma membrane to yield cytoplasmic ceramide, which initiates a cascade of events resulting in different physiological changes (5, 7). In the present study, we were interested in determining if extracellular nSMase signals to induce the expression of Gb3 and the recently characterized glycosyltransferase genes of the Gb3 biosynthetic pathway (6, 9).
MATERIALS AND METHODS
Reagents.
We used the following reagents: cell culture medium, l-glutamine, and fetal bovine serum (Gibco-BRL, Grand Island, N.Y.); hydrocortisone, epidermal growth factor, Staphylococcus aureus SMase, and EGTA (Sigma Chemical Co., St. Louis, Mo.); TNF-α (Boehringer Mannheim, Indianapolis, Ind.); and [γ-32P]ATP (New England Nuclear Corp., Boston, Mass.). Stx2 was immunoaffinity purified from a clinical isolate of EHEC.
Cell lines and culture conditions.
Human dermal microvascular endothelial cells (HDMEC) were obtained from the Centers for Disease Control (Atlanta, Ga.). They are simian virus 40 T-antigen-transformed cells that have been characterized as having the endothelial phenotype (1, 37). HDMEC were maintained in MCDB 131 medium containing 1 μg of hydrocortisone/ml, 10 ng of epidermal growth factor/ml, 300 μg of l-glutamine/ml, and 15% fetal bovine serum at 37°C in a 5% CO2 atmosphere.
Cell viability assay.
HDMEC were dispensed into 96-well culture plates at a density of 15,000 cells per well. After the plates were incubated overnight, inducers were added to the plates at the concentrations and for the times indicated in the figure legends and figures. The inducers were removed, and Stx2 (0.1 nM) was added for 24 h, followed by determination of cell viability by the neutral red assay as previously described (16). Cells maintained in medium alone served as the 100%-viability control. The data are the means of results from triplicate wells.
Gb3 analysis.
Cells were grown to confluence in T75 flasks and treated with medium alone or SMase (0.067 U/ml) as indicated in the figures. After incubation, the cells were washed twice in cold phosphate-buffered saline, removed from the flask with an EGTA solution, pelleted at 2,600 rpm at 4°C, and frozen at −20°C. Glycolipid isolation, analysis by thin-layer chromatography, and quantitation by high-performance liquid chromatography (HPLC) were performed as previously described (35).
Ceramide assay.
Cells were grown to confluence in T150 flasks and treated with SMase (0.067 U/ml) or TNF-α (200 U/ml) for up to 48 h. Control cells were treated with media only. The cells were then washed twice with prewarmed phosphate-buffered saline, removed from the flasks with EGTA solution, and pelleted at 6,000 rpm at 4°C. The pellet was resuspended in 2 ml of methanol, transferred to a glass tube, and stored at −85°C. Total neutral sphingolipids were extracted with organic solvents. The lipids were then radiolabeled with 32P by diacylglycerol kinase prior to resolution by thin-layer chromatography by using a previously described methodology (3, 24).
Northern blot analysis.
The total RNA was isolated from cells by using RNeasy columns according to the instructions of the manufacturer (Qiagen, Valencia, Calif.) and quantified at 260 nm. Ten micrograms of RNA was separated on a 1% formaldehyde-agarose gel and transferred by capillary action to a nylon membrane (Hybond-N; Amersham). RNA was cross-linked to the membrane with a UV Stratalinker (Stratagene). Blots were prehybridized for 4 h (for ceramide:glucosyltransferase [CGT] and 18S probes) or overnight (for the Gb3 synthase probe) at 42°C in either homemade buffer containing 5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]), 50% formamide, 5× Denhardt's solution, 0.5% sodium dodecyl sulfate (SDS), and 10 μg of salmon sperm DNA per ml or ULTRAhyb buffer (Ambion, Austin, Tex.). Blots were then hybridized overnight at 42°C in the same solution containing the labeled probes.
A 450-bp CGT-specific probe was derived as previously described by PCR amplification from a clone containing the human CGT cDNA (strain 530753) obtained from the American Type Culture Collection (Manassas, Va.). It was labeled with [32P]dATP by using a random primer kit (BRL Life Technologies) according to the manufacturer's protocol. The Gb3 synthase probe was synthesized from mouse kidney RNA by reverse transcription-PCR with primers based on the rat sequence (GenBank accession no. AF248544). The upstream primer was 5′-TAGCCTCGAGCGTAAGC-3′, and the downstream primer was 5′-CACAAGAATGACCCCACC-3′, which amplified a 714-bp fragment. The purified fragment was labeled overnight by the random primer method with [32P]dATP and a random primer labeling kit (Invitrogen, Carlsbad, Calif.). The denatured probe was added to the prehybridization buffer at 106 cpm/ml for overnight hybridization. The 18S oligonucleotide probe (5′-TATTGGAGCTGGAATTACCGCGGCTGCTGG-3′) was end labeled with [γ-32P]ATP and T4 polynucleotide kinase (BRL Life Technologies). After hybridization, the blots were washed in increasingly stringent SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-SDS buffers to remove background and subjected to autoradiography with X-Omat film (Eastman Kodak). The blots were stripped between probes by being boiled in 0.1% SDS.
Quantitation and normalization of message was performed by photographing the autoradiographs with a Kodak EDAS 290 digital camera and by using 1D Image Analysis software, version 3.5 (Scientific Imaging Systems, Eastman Kodak Company, Rochester, N.Y.). The sum intensity of each band was divided by the sum intensity of its corresponding 18S RNA band, multiplied by 100, and graphed.
RESULTS
SMase induction of sensitivity to Stx2 in endothelial cells.
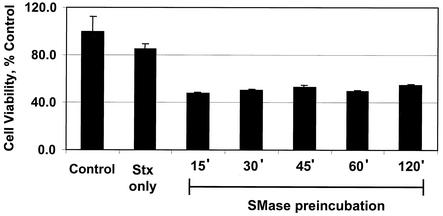
A property of SMase is its capacity to directly interact with HDMEC and induce sensitivity to the Shiga toxins. The kinetics of this induction process is depicted in Fig. 1. The data demonstrate that in comparison to other inducers of Stx sensitivity in endothelial cells, such as bacterial LPS, TNF-α, and IL-1β, SMase was by far the most rapid, with Stx2 sensitivity being noticeable within 1 h (8, 16, 17, 21). SMase increased the receptor quantity in endothelial cells (Table 1), suggesting that this quantity was responsible for the enhanced sensitivity of endothelial cells to Stx2. Within 0.25 h after exposure to SMase, the total Gb3 content of endothelial cells increased twofold. This change in Gb3 content coincided with an increased binding of radiolabeled Stx2 to the cells (data not shown). No further increase in Gb3 or Stx2 binding was observed beyond the 0.5-h time point. We have also documented that SMase induction of Stx2 sensitivity takes place in human umbilical vein endothelial cells. The latter data are the subject of a separate manuscript in progress.
FIG. 1.
Preincubation time course of SMase induction of Stx sensitivity on endothelial cells. HDMEC were preincubated with SMase (0.067 U/ml) for the times indicated. Following the removal of SMase, the cells were incubated with purified Stx2 (0.1 nM) for 24 h, after which cell viability was determined as described in Materials and Methods. Data are presented as the means of results from triplicate samples, and cell viability is expressed as a percentage of the cell viability of the control sample, which was incubated in the absence of SMase and Stx2. These results are representative of results from multiple trials. The incubation of HDMEC with SMase alone did not reduce cell viability.
TABLE 1.
Induction of the Shiga toxin receptor Gb3 on endothelial cellsa
| Preincubation time (h) | SMaseb | Amt of Gb3 (fmol/cell) | Amt of Gb3/amt in control cells (%) |
|---|---|---|---|
| 0 | − | 0.326 | 100 |
| 0.25 | + | 0.644 | 198 |
| 1.0 | + | 0.650 | 199 |
| 4.0 | − | 0.289 | 100 |
| 4.0 | + | 0.533 | 191 |
HDMEC were incubated with SMase (0.067 U/ml) for the times indicated, followed by extraction of the total amount of neutral glycosphingolipids and quantitative analysis of Gb3 by HPLC as described in Materials and Methods. These data are representative of results from duplicate experiments.
+, SMase present; −, SMase not present.
SMase induction of ceramide in HDMEC.
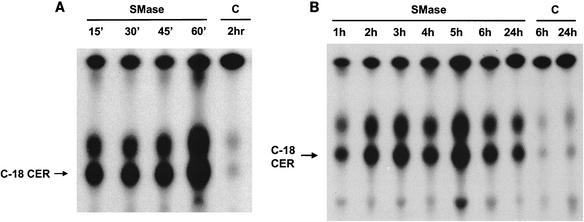
To further define the mechanism of SMase induction of Stx sensitivity in endothelial cells, the biochemical action of SMase was examined. SMase is known to enzymatically generate ceramide from membrane sphingomyelin. Thus, as a measure of SMase activity, ceramide was quantified in endothelial cells following the cells' exposure to SMase as described in Materials and Methods. Total cellular ceramide was 32P radiolabeled with diacylglycerol kinase and [γ-32P]ATP, followed by the separation of labeled lipids with ascending thin-layer chromatography. The resulting autoradiographs (Fig. 2) show that ceramide was barely detectable in untreated endothelial cells. However, SMase treatment resulted in a substantial increase of intracellular ceramide. The increase of ceramide was noticeable as early as 0.25 h (Fig. 2A) after exposure and peaked at 5 h, with a gradual decrease through the remainder of the 24-h time period (Fig. 2B). The two bands of ceramide shown in Fig. 2 represent C18 ceramide and an unidentified, faster-migrating form of ceramide, likely to be either C22 or C24 ceramide. Both bands increased proportionally in response to SMase. Diacylglycerol (upper band) was present in control cells, and its levels did not change substantially following exposure to SMase. It was noted that another inducer of Stx sensitivity, TNF-α, did not change the amount of ceramide in HDMEC, in contrast to the results obtained with SMase-treated cells (data not shown).
FIG. 2.
Induction of ceramide by SMase in HDMEC. HDMEC were incubated with SMase (0.067 U/ml) for shorter (A) or longer (B) periods of time, and cellular ceramide was quantified as described in Materials and Methods. The data are representative of results from four different experiments performed under these conditions. C18 ceramide was undetectable at zero time. C, untreated control cells; C-18 CER, C-18 ceramide.
Regulation of the Gb3 biosynthetic pathway by SMase.
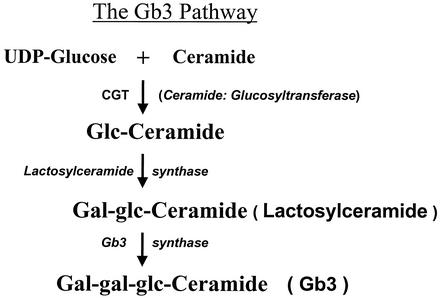
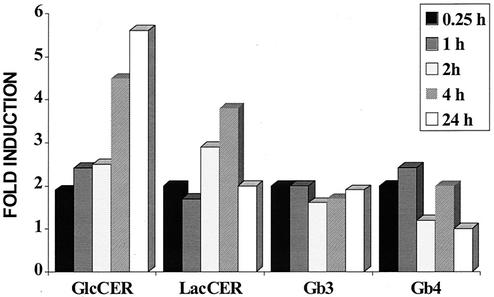
An examination of the effects of SMase on the Gb3 synthetic pathway was undertaken to determine which steps of the Gb3 pathway were being affected by SMase. This was accomplished by monitoring the activity of the three glycosyltransferase enzymes in the Gb3 pathway (Fig. 3). Glycosphingolipid products of the individual glycosyltransferase enzymes were measured in HDMEC following the incubation of HDMEC with SMase for 0.25 to 24 h. The results shown in Fig. 4 indicate that SMase exerted most of its effect on the first glycosyltransferase enzyme in the pathway, CGT. SMase caused a time-dependent increase in glucosylceramide, leading to a maximal, sixfold increase by 24 h. A related but lesser increase was also observed in lactosylceramide (LacCER), the product of LacCER synthase, the second glycosyltransferase enzyme in the Gb3 pathway. Although the amount of Gb3 doubled rapidly after SMase treatment of the cells, the response reached a plateau over the 24-h time period. Finally, little change was noted in the amount of Gb4 (globotetraosylceramide) following treatment of the endothelial cells with SMase, indicating that the increase in Gb3 was not a result of degradative conversion of Gb4 to Gb3. In summary, the data suggest that a further increase in Gb3 was limited by the activity of Gb3 synthase.
FIG. 3.
Scheme of the Gb3 biosynthetic pathway. Depicted are the three enzymes and their respective products of the Gb3 pathway of eukaryotic cells. Gb3 is the primary receptor for Stx1 and Stx2 (11, 31).
FIG. 4.
Induction by SMase of neutral glycosphingolipids of the Gb3 pathway in HDMEC. HDMEC were incubated with SMase (0.067 U/ml) for 0.25, 1, 2, 4, or 24 h. Total neutral glycosphingolipids were extracted and analyzed by HPLC as described in Materials and Methods. The data shown are representative of results from duplicate experiments. GlcCER, glucosylceramide.
Induction of Gb3 pathway glycosyltransferase enzymes by SMase.
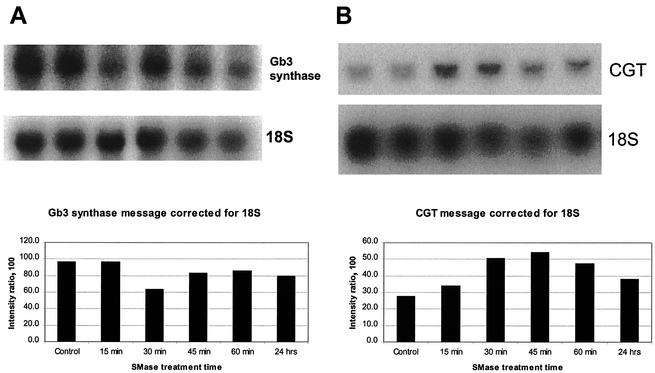
We sought to determine if intracellular ceramide generated by SMase could function by increasing transcription of the CGT gene in HDMEC. To examine this possibility, the relative amount of mRNA for this enzyme was measured in HDMEC after treatment of the cells with SMase. Control untreated HDMEC produced a detectable amount of CGT mRNA (Fig. 5B). A significant increase in the amount of CGT mRNA was observed within 30 min after the exposure of HDMEC to SMase, and the amount had almost doubled at 45 min and remained elevated by 35% at 24 h. This timing of CGT mRNA induction coincided with the early increase in intracellular ceramide (Fig. 2A) and glucosylceramide (Fig. 4) following SMase treatment of HDMEC.
FIG. 5.
Effect of SMase on induction of the Gb3 biosynthetic pathway enzymes Gb3 synthase (A) and CGT (B) in HDMEC. HDMEC were incubated with SMase (0.067 U/ml) for the times indicated, the total RNA was isolated, and Gb3 synthase mRNA (A) or CGT mRNA (B) was measured as described in Materials and Methods. The data shown are representative of results from triplicate experiments. The intensities of the mRNA bands were normalized to that of the 18S RNA loading. Control, without SMase treatment.
Transcriptional regulation of Gb3 synthase was also examined. HDMEC constitutively expressed Gb3 synthase mRNA in a relatively large amount (Fig. 5). SMase actually decreased this level of mRNA in a transient manner (Fig. 5), and an increase in Gb3 synthase mRNA was not observed over the entire time course. Again, these Gb3 mRNA results were in agreement with the amount of Gb3 produced in SMase-treated HDMEC (Fig. 4). These data suggested that the Gb3 synthase enzyme was rate limiting for Gb3 synthesis in HDMEC exposed to SMase. Further support of this concept was that LacCER accumulated at levels up to fourfold the normal level at 4 h but appeared not to be converted to Gb3 (Fig. 4).
DISCUSSION
The present study demonstrates that bacterial nSMase sensitized human endothelial cells to Shiga toxin. This action of SMase is rapid, occurring within minutes, and as such differs from those of other inducers of Stx sensitivity, including TNF-α, IL-1β, and bacterial LPS, all of which require 24 to 48 h to maximally sensitize endothelial cells to the Shiga toxins (8, 16, 32, 36). Sensitization of endothelial cells to Stx2 by bacterial SMase is accompanied within 30 min by an increase of intracellular ceramide, most likely derived from plasma membrane sphingomyelin.
Although ceramide has been implicated in different cellular responses, including apoptosis, this is the first report of SMase induction of Stx2 sensitivity and of the Stx2 receptor, the neutral glycosphingolipid Gb3. The data indicate that SMase induces Gb3 synthesis by transcriptional activation of the gene encoding CGT, the first glycosyltransferase in the Gb3 pathway. However, it is also possible that ceramide substrate limitation is in part responsible for the observed increase in Gb3, because ceramide is a component of Gb3. Induction of both the ceramide:glucosylceramide transferase gene and its product strongly suggests that control occurs at this level. Gb3 synthase mRNA encoded by the gene for the third enzyme of the Gb3 synthetic pathway is constitutively activated in HDMEC. SMase does not alter the level of Gb3 synthase mRNA under conditions where the CGT mRNA is markedly increased. Together, these results suggest that the rate-limiting step in Gb3 synthesis in HDMEC lies at the level of CGT rather than at that of the Gb3 synthase.
The natural source of nSMase in the setting of a systemic EHEC response is somewhat speculative, but multiple sources of nSMase do exist. A search of the E. coli O157:H7 genome database (University of Wisconsin) did not detect an SMase gene. Although it is possible that SMase is derived from intestinal bacteria and enters the blood during the hemorrhagic-colitis phase of EHEC disease, more plausible sources exist within the host. For example, nSMase is released from activated platelets and endothelial cells (26-28, 30). It is noteworthy that although intracellular ceramide has been associated with apoptosis in some cells, SMase did not cause apoptosis of HDMEC in the present study. Additional work is ongoing to determine the fate of the ceramide induced by SMase in HDMEC, as such information should provide useful clues regarding the regulation of Gb3 synthesis and enhanced Stx2 sensitivity.
Acknowledgments
We appreciate the assistance of Donald Kohan for information related to the Gb3 experiments.
This work was supported by Public Health Service grant AI-24431 from the National Institute of Allergy and Infectious Diseases.
Editor: D. L. Burns
REFERENCES
- 1.Ades, E. W., F. J. Candal, R. A. Swerlick, V. G. George, S. Summers, D. C. Bosse, and T. J. Lawley. 1992. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 99:683-690. [DOI] [PubMed] [Google Scholar]
- 2.Altura, B. M., A. Gebrewold, T. Zheng, and B. T. Altura. 2002. Sphingomyelinase and ceramide analogs induce vasoconstriction and leukocyte-endothelial interactions in cerebral venules in the intact rat brain: insight into mechanisms and possible relation to brain injury and stroke. Brain Res. Bull. 58:271-278. [DOI] [PubMed] [Google Scholar]
- 3.Bielawska, A., D. K. Perry, and Y. A. Hannun. 2001. Determination of ceramides and diglycerides by the diglyceride kinase assay. Anal. Biochem. 298:141-150. [DOI] [PubMed] [Google Scholar]
- 4.Dbaibo, G. S., W. El-Assaad, A. Krikorian, B. Liu, K. Diab, N. Z. Idriss, M. El-Sabban, T. A. Driscoll, D. K. Perry, and Y. A. Hannun. 2001. Ceramide generation by two distinct pathways in tumor necrosis factor alpha-induced cell death. FEBS Lett. 503:7-12. [DOI] [PubMed] [Google Scholar]
- 5.Hannun, Y. A. 1996. Functions of ceramide in coordinating cellular responses to stress. Science 274:1855-1859. [DOI] [PubMed] [Google Scholar]
- 6.Ichikawa, S., K. Ozawa, and Y. Hirabayashi. 1997. Assignment of a UDP-glucose:ceramide glucosyltransferase gene (UGCG) to human chromosome band 9q31 by in situ hybridization. Cytogenet. Cell Genet. 79:233-234. [DOI] [PubMed] [Google Scholar]
- 7.Joseph, C. K., H. S. Byun, R. Bittman, and R. N. Kolesnick. 1993. Substrate recognition by ceramide-activated protein kinase. Evidence that kinase activity is proline-directed. J. Biol. Chem. 268:20002-20006. [PubMed] [Google Scholar]
- 8.Kaye, S. A., C. B. Louise, B. Boyd, C. A. Lingwood, and T. G. Obrig. 1993. Shiga toxin-associated hemolytic uremic syndrome: interleukin-1β enhancement of Shiga toxin cytotoxicity toward human vascular endothelial cells in vitro. Infect. Immun. 61:3886-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keusch, J. J., S. M. Manzella, K. A. Nyame, R. D. Cummings, and J. U. Baenziger. 2000. Cloning of Gb3 synthase, the key enzyme in globo-series glycosphingolipid synthesis, predicts a family of alpha 1,4-glycosyltransferases conserved in plants, insects, and mammals. J. Biol. Chem. 275:25315-25321. [DOI] [PubMed] [Google Scholar]
- 10.Kiarash, A., B. Boyd, and C. A. Lingwood. 1994. Glycosphingolipid receptor function is modified by fatty acid content. Verotoxin 1 and verotoxin 2c preferentially recognize different globotriaosyl ceramide fatty acid homologues. J. Biol. Chem. 269:11138-11146. [PubMed] [Google Scholar]
- 11.Lingwood, C. A. 1996. Role of verotoxin receptors in pathogenesis. Trends Microbiol. 4:147-153. [DOI] [PubMed] [Google Scholar]
- 12.Lingwood, C. A. 1993. Verotoxins and their glycolipid receptors. Adv. Lipid Res. 25:189-211. [PubMed] [Google Scholar]
- 13.Liu, B., N. Andrieu-Abadie, T. Levade, P. Zhang, L. M. Obeid, and Y. A. Hannun. 1998. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-alpha-induced cell death. J. Biol. Chem. 273:11313-11320. [DOI] [PubMed] [Google Scholar]
- 14.Liu, B., L. M. Obeid, and Y. A. Hannun. 1997. Sphingomyelinases in cell regulation. Semin. Cell Dev. Biol. 8:311-322. [DOI] [PubMed] [Google Scholar]
- 15.Louise, C. B., and T. G. Obrig. 1994. Human renal microvascular endothelial cells as a potential target in the development of the hemolytic uremic syndrome as related to fibrinolysis factor expression, in vitro. Microvasc. Res. 47:377-387. [DOI] [PubMed] [Google Scholar]
- 16.Louise, C. B., and T. G. Obrig. 1992. Shiga toxin-associated hemolytic uremic syndrome: combined cytotoxic effects of Shiga toxin and lipopolysaccharide (endotoxin) on human vascular endothelial cells in vitro. Infect. Immun. 60:1536-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louise, C. B., and T. G. Obrig. 1991. Shiga toxin-associated hemolytic-uremic syndrome: combined cytotoxic effects of Shiga toxin, interleukin-1β, and tumor necrosis factor alpha on human vascular endothelial cells in vitro. Infect. Immun. 59:4173-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louise, C. B., M. C. Tran, and T. G. Obrig. 1997. Sensitization of human umbilical vein endothelial cells to Shiga toxin: involvement of protein kinase C and NF-κB. Infect. Immun. 65:3337-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien, A. D., V. L. Tesh, A. Donohue-Rolfe, M. P. Jackson, S. Olsnes, K. Sandvig, A. A. Lindberg, and G. T. Keusch. 1992. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 180:65-94. [DOI] [PubMed] [Google Scholar]
- 20.Obrig, T. G., P. J. Del Vecchio, J. E. Brown, T. P. Moran, B. M. Rowland, T. K. Judge, and S. W. Rothman. 1988. Direct cytotoxic action of Shiga toxin on human vascular endothelial cells. Infect. Immun. 56:2373-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obrig, T. G., C. B. Louise, C. A. Lingwood, B. Boyd, L. Barley-Maloney, and T. O. Daniel. 1993. Endothelial heterogeneity in Shiga toxin receptors and responses. J. Biol. Chem. 268:15484-15488. [PubMed] [Google Scholar]
- 22.Perry, D. K., and Y. A. Hannun. 1998. The role of ceramide in cell signaling. Biochim. Biophys. Acta 1436:233-243. [DOI] [PubMed] [Google Scholar]
- 23.Pickering, L. K., T. G. Obrig, and F. B. Stapleton. 1994. Hemolytic-uremic syndrome and enterohemorrhagic Escherichia coli. Pediatr. Infect. Dis. J. 13:459-476. [DOI] [PubMed] [Google Scholar]
- 24.Preiss, J., C. R. Loomis, W. R. Bishop, R. Stein, J. E. Niedel, and R. M. Bell. 1986. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J. Biol. Chem. 261:8597-8600. [PubMed] [Google Scholar]
- 25.Ramegowda, B., and V. L. Tesh. 1996. Differentiation-associated toxin receptor modulation, cytokine production, and sensitivity to Shiga-like toxins in human monocytes and monocytic cell lines. Infect. Immun. 64:1173-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romiti, E., E. Meacci, M. Tani, F. Nuti, M. Farnararo, M. Ito, and P. Bruni. 2000. Neutral/alkaline and acid ceramidase activities are actively released by murine endothelial cells. Biochem. Biophys. Res. Commun. 275:746-751. [DOI] [PubMed] [Google Scholar]
- 27.Romiti, E., E. Meacci, G. Tanzi, L. Becciolini, S. Mitsutake, M. Farnararo, M. Ito, and P. Bruni. 2001. Localization of neutral ceramidase in caveolin-enriched light membranes of murine endothelial cells. FEBS Lett. 506:163-168. [DOI] [PubMed] [Google Scholar]
- 28.Romiti, E., V. Vasta, E. Meacci, M. Farnararo, T. Linke, K. Ferlinz, K. Sandhoff, and P. Bruni. 2000. Characterization of sphingomyelinase activity released by thrombin-stimulated platelets. Mol. Cell. Biochem. 205:75-81. [DOI] [PubMed] [Google Scholar]
- 29.Ruggenenti, P., M. Noris, and G. Remuzzi. 2001. Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int. 60:831-846. [DOI] [PubMed] [Google Scholar]
- 30.Simon, C. G., Jr., S. Chatterjee, and A. R. Gear. 1998. Sphingomyelinase activity in human platelets. Thromb. Res. 90:155-161. [DOI] [PubMed] [Google Scholar]
- 31.Soltyk, A. M., C. R. MacKenzie, V. M. Wolski, T. Hirama, P. I. Kitov, D. R. Bundle, and J. L. Brunton. 2002. A mutational analysis of the globotriaosylceramide-binding sites of verotoxin VT1. J. Biol. Chem. 277:5351-5359. [DOI] [PubMed] [Google Scholar]
- 32.Tesh, V. L. 1998. Virulence of enterohemorrhagic Escherichia coli: role of molecular crosstalk. Trends Microbiol. 6:228-233. [DOI] [PubMed] [Google Scholar]
- 33.Tesh, V. L., and A. D. O'Brien. 1991. The pathogenic mechanisms of Shiga toxin and the Shiga-like toxins. Mol. Microbiol. 5:1817-1822. [DOI] [PubMed] [Google Scholar]
- 34.Tesh, V. L., B. Ramegowda, and J. E. Samuel. 1994. Purified Shiga-like toxins induce expression of proinflammatory cytokines from murine peritoneal macrophages. Infect. Immun. 62:5085-5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ullman, M. D., and R. H. McCluer. 1977. Quantitative analysis of plasma neutral glycosphingolipids by high performance liquid chromatography of their perbenzoyl derivatives. J. Lipid Res. 18:371-378. [PubMed] [Google Scholar]
- 36.van de Kar, N. C., L. A. Monnens, M. A. Karmali, and V. W. van Hinsbergh. 1992. Tumor necrosis factor and interleukin-1 induce expression of the verocytotoxin receptor globotriaosylceramide on human endothelial cells: implications for the pathogenesis of the hemolytic uremic syndrome. Blood 80:2755-2764. [PubMed] [Google Scholar]
- 37.Xu, Y., R. A. Swerlick, N. Sepp, D. Bosse, E. W. Ades, and T. J. Lawley. 1994. Characterization of expression and modulation of cell adhesion molecules on an immortalized human dermal microvascular endothelial cell line (HMEC-1). J. Investig. Dermatol. 102:833-837. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, P., B. Liu, G. M. Jenkins, Y. A. Hannun, and L. M. Obeid. 1997. Expression of neutral sphingomyelinase identifies a distinct pool of sphingomyelin involved in apoptosis. J. Biol. Chem. 272:9609-9612. [DOI] [PubMed] [Google Scholar]