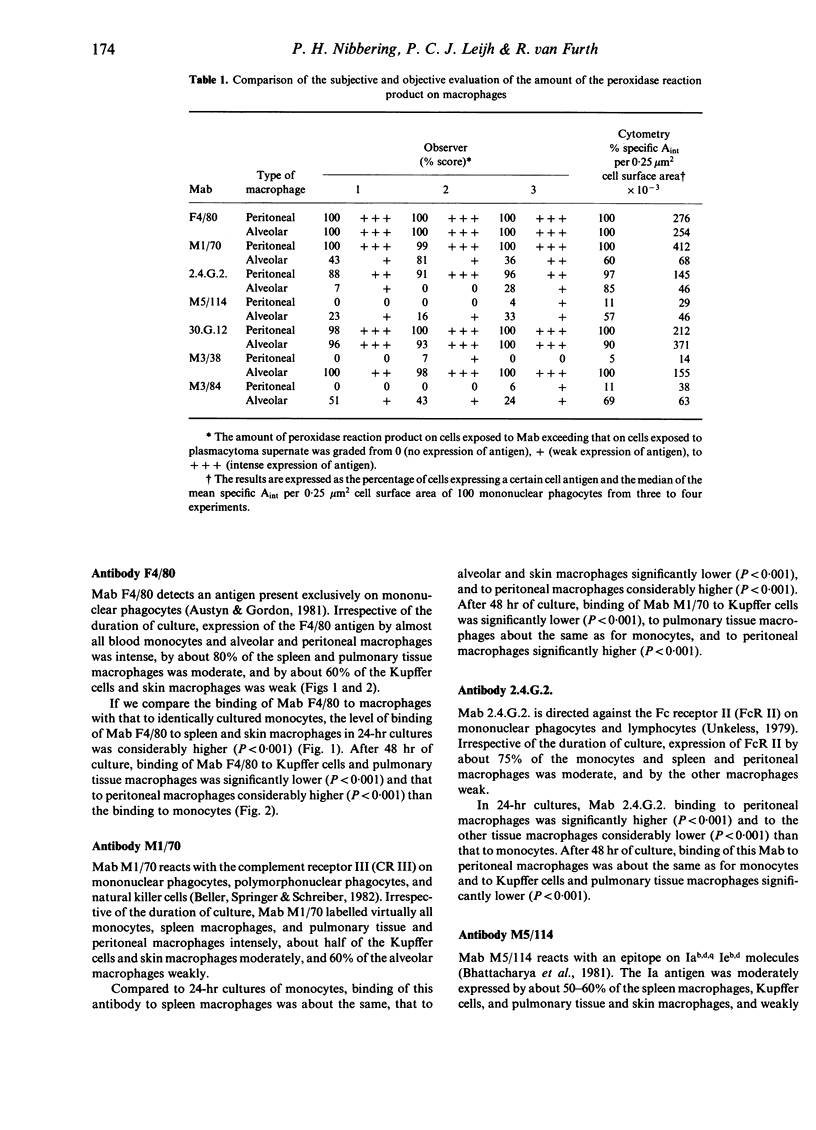
Abstract
The purpose of the present study was to compare the monoclonal antibody (Mab) binding patterns of various tissue macrophages with each other and with blood monocytes. To allow recovery from the effects of the isolation procedure, or to obtain purified populations, macrophages were cultured for 24 hr and 48 hr. For comparison, blood monocytes were also cultured for 24 hr and 48 hr. Mab binding to individual cells, detected by the biotin avidin immunoperoxidase method, was quantified cytophotometrically and the results expressed as the median of the specific mean absorbance per 0.25 micron2 cell surface area or as specific integrated absorbance per cell. Analysis of the quantitative data in relation to the results of subjective evaluation of the peroxidase reaction product, demonstrating Mab binding to cells, yielded three classes for description of the intensity of antigen expression by cells: weak (specific mean absorbance per unit cell surface less than 0.07), moderate (values between 0.07 and 0.14), and intense (values more than 0.14). No matter how the results were expressed, comparison of the Mab binding patterns of macrophages with those of blood monocytes showed that spleen macrophages bound significantly less F4/80 and more M5/114 (Ia antigen). Kupffer cells and skin macrophages bound either approximately the same amount or considerably less of the various Mabs than monocytes did. Pulmonary tissue and alveolar macrophages bound significantly more 30.G.12 (leucocyte antigen), M3/38 (Mac-2 antigen), and M3/84 (Mac-3 antigen) and comparable amounts or considerably less of the other Mabs than the monocytes did. Peritoneal macrophages bound significantly more F4/80, M1/70 (complement receptor III), and 2.4.G.2. (Fc receptor II) and comparable amounts or considerably less of the other Mabs than monocytes did. It is concluded that macrophages from different organs and different anatomical sites within one organ differ from one another, for example, peritoneal macrophages do not resemble any other population of macrophages and alveolar macrophages do not resemble pulmonary tissue macrophages, and differentiation of blood monocytes into tissue macrophages does not show a distinct pattern.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Calamai E. G., Beller D. I., Unanue E. R. Regulation of macrophage populations. IV. Modulation of Ia expression in bone marrow-derived macrophages. J Immunol. 1982 Apr;128(4):1692–1694. [PubMed] [Google Scholar]
- Ho M. K., Springer T. A. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol. 1982 Mar;128(3):1221–1228. [PubMed] [Google Scholar]
- Ho M. K., Springer T. A. Tissue distribution, structural characterization, and biosynthesis of Mac-3, a macrophage surface glycoprotein exhibiting molecular weight heterogeneity. J Biol Chem. 1983 Jan 10;258(1):636–642. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Nibbering P. H., Leijh P. C., van Furth R. Quantitative immunocytochemical characterization of mononuclear phagocytes. I. Monoblasts, promonocytes, monocytes, and peritoneal and alveolar macrophages. Cell Immunol. 1987 Apr 1;105(2):374–385. doi: 10.1016/0008-8749(87)90085-2. [DOI] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M., Unkeless J. C., Witmer M. D., Gutchinov B., Cohn Z. A. Studies of the cell surface of mouse dendritic cells and other leukocytes. J Exp Med. 1981 Jul 1;154(1):168–187. doi: 10.1084/jem.154.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg M., Van den Broek K., Smeulders A. W., Vossepoel A. M., Van Duijn P. HIDACSYS: computer programs for interactive scanning cytophotometry. Histochemistry. 1977 Dec 28;54(4):273–288. doi: 10.1007/BF00508271. [DOI] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Nibbering P. H., van Dissel J. T., Diesselhoff-den Dulk M. M. The characterization, origin, and kinetics of skin macrophages during inflammation. J Invest Dermatol. 1985 Nov;85(5):398–402. doi: 10.1111/1523-1747.ep12277056. [DOI] [PubMed] [Google Scholar]
- van oud Alblas A. B., van Furth R. Origin, Kinetics, and characteristics of pulmonary macrophages in the normal steady state. J Exp Med. 1979 Jun 1;149(6):1504–1518. doi: 10.1084/jem.149.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]



