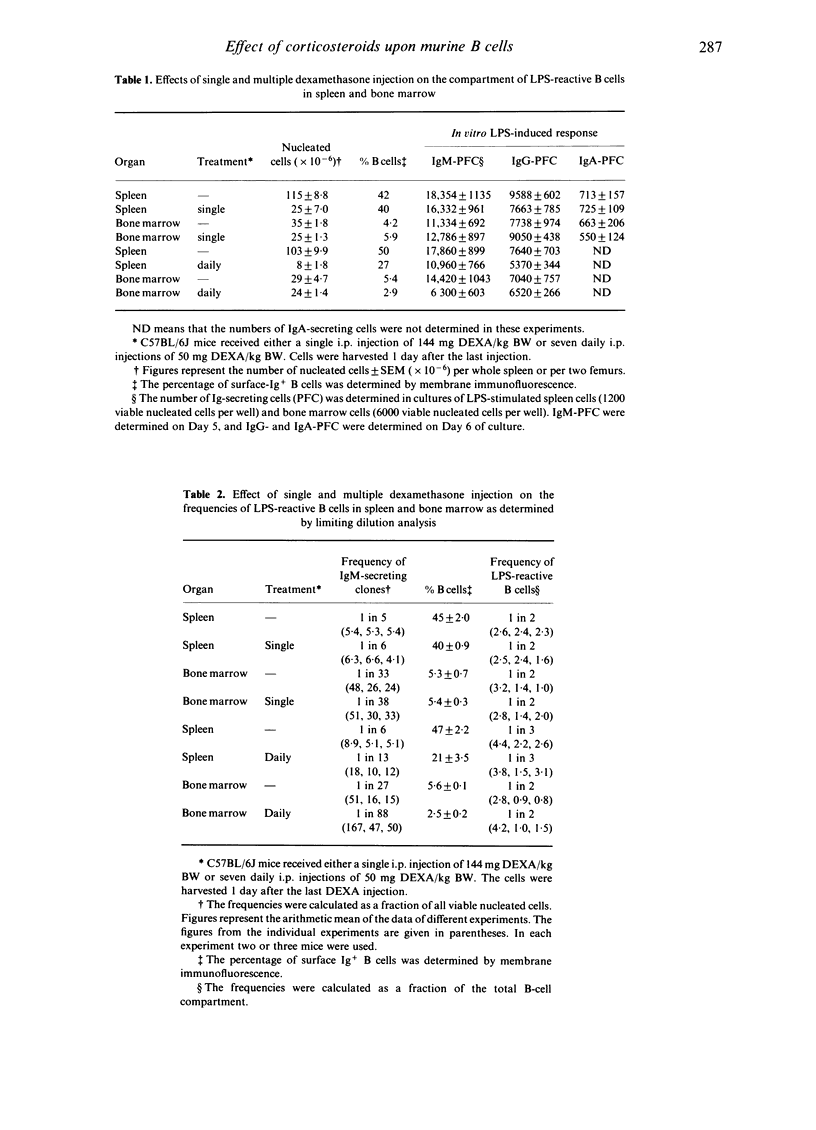
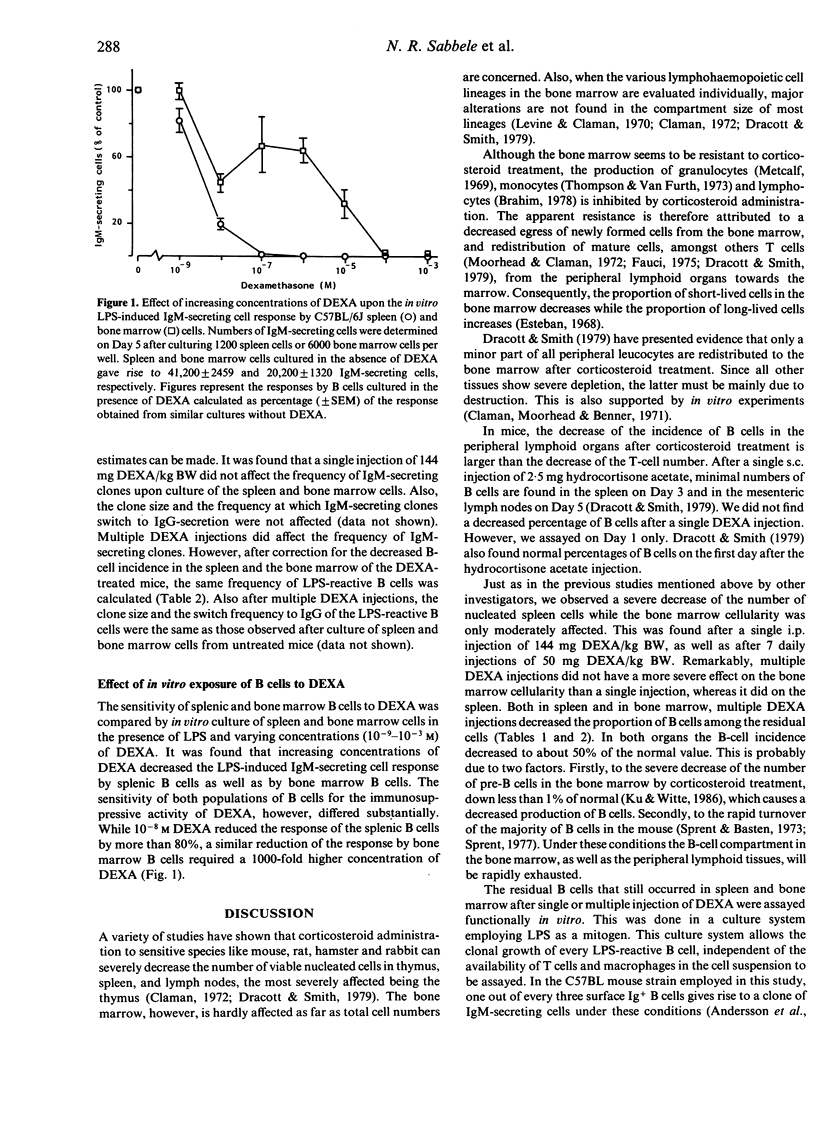
Abstract
The influence of the synthetic corticosteroid dexamethasone sodium phosphate (DEXA) upon mouse B cells was studied. This was done by in vivo treatment of mice with a single or multiple injection of DEXA, and by culturing murine spleen cells and bone marrow cells in vitro in the presence of different concentrations of DEXA. The effect of DEXA on the B-cell compartment was assayed by polyclonal stimulation of the B cells by Escherichia coli lipopolysaccharide (LPS) in vitro and subsequent measurement of the Ig-secreting cell response in the protein A plaque assay. DEXA treatment could greatly reduce the number of B cells in the spleen, but the bone marrow B-cell compartment was quite resistant to DEXA. The in vitro LPS-induced IgM response of the residual B cells from both spleen and bone marrow and their capacity to switch from IgM to IgG and IgA secretion were not affected. These data indicate that DEXA can decrease the total number of B cells but not the functional capacity of the residual LPS-reactive B cells. This was confirmed at the clonal level by limiting dilution culture experiments. The contrasting effects of DEXA on splenic and bone marrow B cells was also found when the cells were exposed to the drug in vitro. It was found that 10(-8) M DEXA in vitro reduced the response of splenic B cells to LPS by more than 80%, while a similar reduction of the response by bone marrow B cells required a 1000-fold higher concentration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Coutinho A., Melchers F. Frequencies of mitogen-reactive B cells in the mouse. I. Distribution in different lymphoid organs from different inbred strains of mice at different ages. J Exp Med. 1977 Jun 1;145(6):1511–1519. doi: 10.1084/jem.145.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner R., Coutinho A., Rijnbeek A. M., van Oudenaren A., Hooijkaas H. Immunoglobulin isotype expression. II. Frequency analysis in mitogen-reactive B cells. Eur J Immunol. 1981 Oct;11(10):799–804. doi: 10.1002/eji.1830111012. [DOI] [PubMed] [Google Scholar]
- Benner R., van Dongen J. J., van Oudenaren A. Corticosteroids and the humoral immune response of mice i. Differential effect of corticosteroids upon antibody formation to sheep red blood cells in spleen and bone marrow. Cell Immunol. 1978 Nov;41(1):52–61. doi: 10.1016/s0008-8749(78)80027-6. [DOI] [PubMed] [Google Scholar]
- Benner R., van Oudenaren A. Corticosteroids and the humoral immune response of mice. II. Enhancement of bone marrow antibody formation to lipopolysaccharide by high doses of corticosteroids. Cell Immunol. 1979 Dec;48(2):267–275. doi: 10.1016/0008-8749(79)90121-7. [DOI] [PubMed] [Google Scholar]
- Bradley L. M., Mishell R. I. Differential effects of glucocorticosteroids on the functions of helper and suppressor T lymphocytes. Proc Natl Acad Sci U S A. 1981 May;78(5):3155–3159. doi: 10.1073/pnas.78.5.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahim F. Marrow lymphocyte production during chronic hydrocortisone administration. J Reticuloendothel Soc. 1978 Feb;23(2):111–118. [PubMed] [Google Scholar]
- Claman H. N. Corticosteroids and lymphoid cells. N Engl J Med. 1972 Aug 24;287(8):388–397. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Moorhead J. W., Benner W. H. Corticosteroids and lymphoid cells in vitro. I. Hydrocortisone lysis of human, guinea pig, and mouse thymus cells. J Lab Clin Med. 1971 Oct;78(4):499–507. [PubMed] [Google Scholar]
- Coutinho A., Forni L. Intraclonal diversification in immunoglobulin isotype secretion: an analysis of switch probabilities. EMBO J. 1982;1(10):1251–1257. doi: 10.1002/j.1460-2075.1982.tb00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracott B. N., Smith C. E. Hydrocortisone and the antibody response in mice. I. Correlations between serum cortisol levels and cell numbers in thymus, spleen, marrow and lymph nodes. Immunology. 1979 Oct;38(2):429–435. [PMC free article] [PubMed] [Google Scholar]
- Esteban J. N. The differential effect of hydrocortisone on the short-lived small lymphocyte. Anat Rec. 1968 Nov;162(3):349–356. doi: 10.1002/ar.1091620309. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. Mechanisms of corticosteroid action on lymphocyte subpopulations. I. Redistribution of circulating T and b lymphocytes to the bone marrow. Immunology. 1975 Apr;28(4):669–680. [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Mechanisms of corticosteroid action on lymphocyte subpopulations. IV. Effects of in vitro hydrocortisone on naturally occuring and mitogen-induced suppressor cells in man. Cell Immunol. 1979 Apr;44(1):157–168. doi: 10.1016/0008-8749(79)90036-4. [DOI] [PubMed] [Google Scholar]
- Hooijkaas H., Preesman A. A., Benner R. Low dose X-irradiation of thymus filler cells in limiting dilution cultures of LPS-reactive B cells reduces the background Ig-secreting cells without affecting growth-supporting capacity. J Immunol Methods. 1982;51(3):323–330. doi: 10.1016/0022-1759(82)90399-4. [DOI] [PubMed] [Google Scholar]
- Ku G., Witte O. N. Corticosteroid-resistant bone marrow-derived B lymphocyte progenitor for long term in vitro cultures. J Immunol. 1986 Nov 1;137(9):2802–2807. [PubMed] [Google Scholar]
- Levine M. A., Claman H. N. Bone marrow and spleen: dissociation of immunologic properties by cortisone. Science. 1970 Mar 13;167(3924):1515–1517. doi: 10.1126/science.167.3924.1515. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Cortisone action on serum colony-stimulating factor and bone marrow in vitro colony-forming cells. Proc Soc Exp Biol Med. 1969 Oct;132(1):391–394. doi: 10.3181/00379727-132-34222. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Lucas A., Mishell B. B. The role of activated accessory cells in preventing immunosuppression by hydrocortisone. J Immunol. 1977 Jul;119(1):118–122. [PubMed] [Google Scholar]
- Moorhead J. W., Claman H. N. Thymus-derived lymphocytes and hydrocortisone: identification of subsets of theta-bearing cells and redistribution to bone marrow. Cell Immunol. 1972 Sep;5(1):74–86. doi: 10.1016/0008-8749(72)90085-8. [DOI] [PubMed] [Google Scholar]
- Sabbele N. R., van Oudenaren A., Benner R. The effect of corticosteroids upon the number and organ distribution of "background" immunoglobulin-secreting cells in mice. Cell Immunol. 1983 Apr 15;77(2):308–317. doi: 10.1016/0008-8749(83)90031-x. [DOI] [PubMed] [Google Scholar]
- Sprent J., Basten A. Circulating T and B lymphocytes of the mouse. II. Lifespan. Cell Immunol. 1973 Apr;7(1):40–59. doi: 10.1016/0008-8749(73)90181-0. [DOI] [PubMed] [Google Scholar]
- Thompson J., van Furth R. The effect of glucocorticosteroids on the proliferation and kinetics of promonocytes and monocytes of the bone marrow. J Exp Med. 1973 Jan 1;137(1):10–21. doi: 10.1084/jem.137.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ewijk W. Immunohistology of lymphoid and non-lymphoid cells in the thymus in relation to T lymphocyte differentiation. Am J Anat. 1984 Jul;170(3):311–330. doi: 10.1002/aja.1001700307. [DOI] [PubMed] [Google Scholar]
- Van Oudenaren A., Hooijkaas H., Benner R. Improvement of the protein A plaque assay for immunoglobulin secreting cells by using immunoglobulin-depleted guinea pig serum as a source of complement. J Immunol Methods. 1981;43(2):219–224. doi: 10.1016/0022-1759(81)90026-0. [DOI] [PubMed] [Google Scholar]



