Abstract
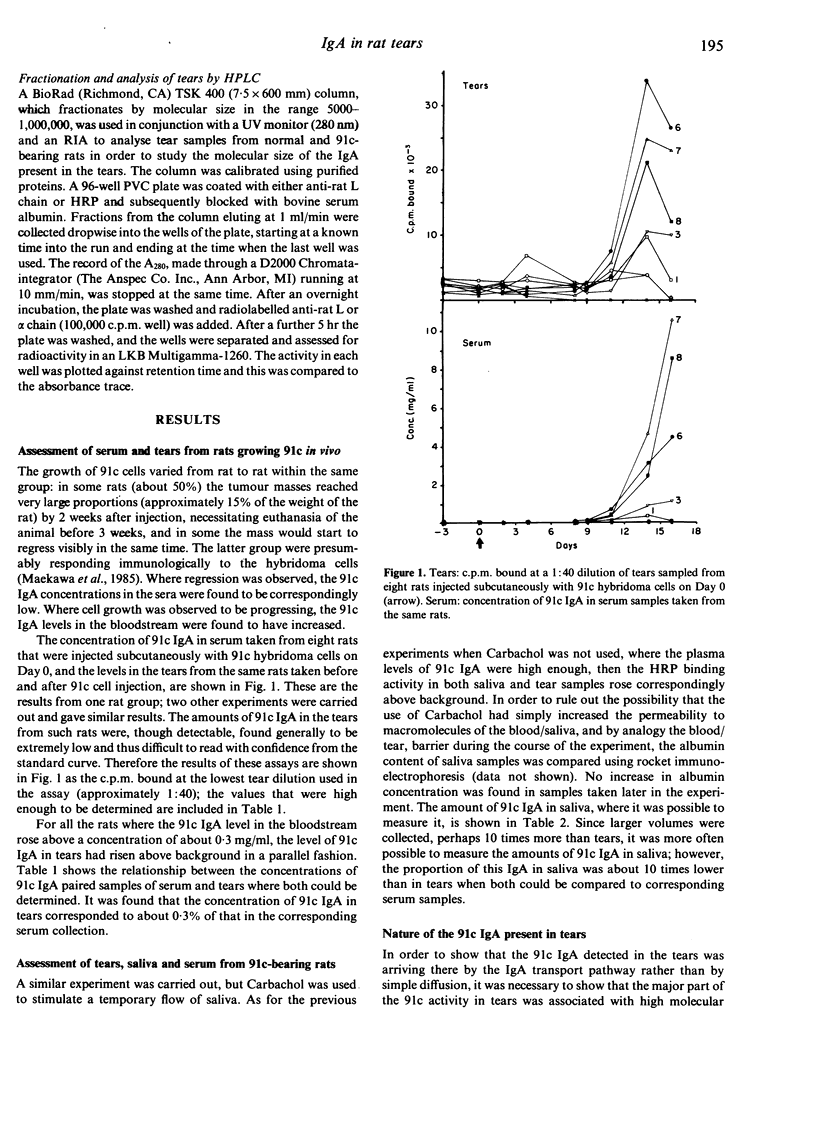
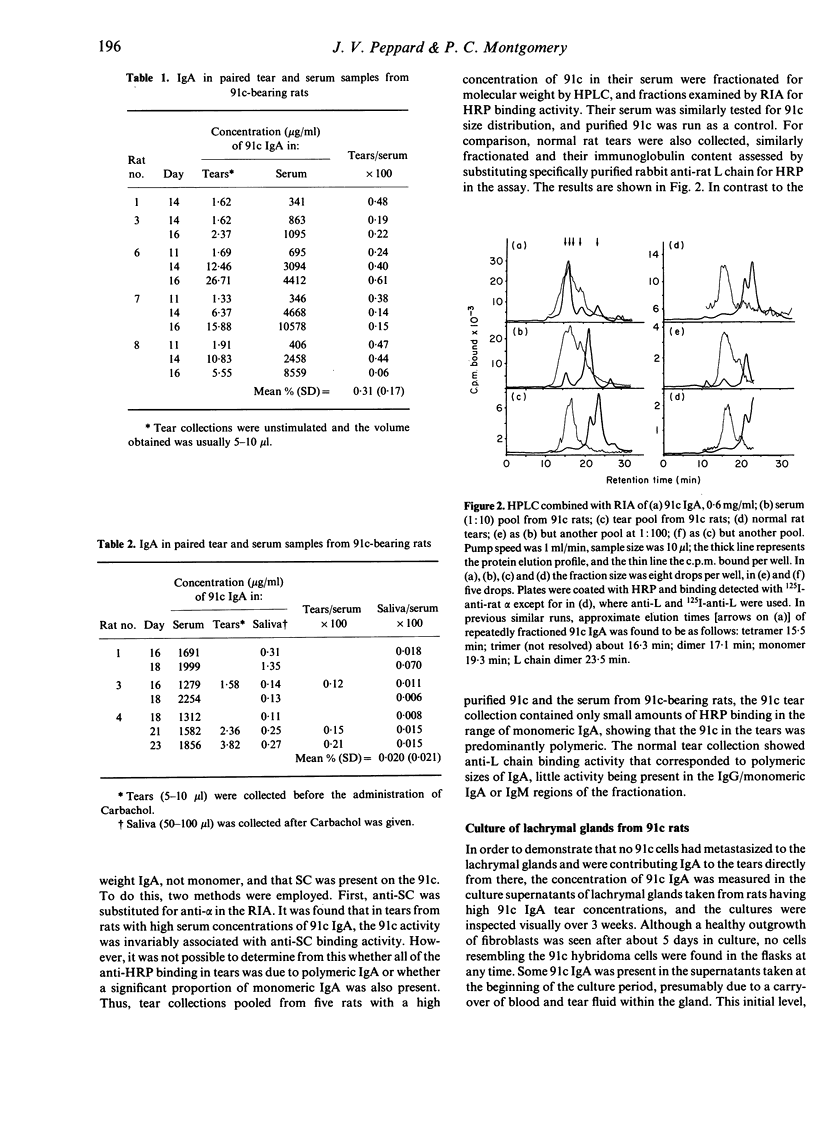
Experiments were carried out to quantify the proportion of IgA in rat tears that arrives from the circulating IgA pool in the blood, as opposed to that supplied by local synthesis in the lacrymal gland. Rats were injected subcutaneously with an IgA-secreting rat hybridoma cell line (91c), which was allowed to grow in vivo, in order to raise the levels of plasma IgA of known antibody specificity. In rats where serum 91c IgA concentrations had built up to 0.3 mg/ml or more, unstimulated tears contained 91c antibody activity of a molecular weight range that corresponded to that of the polymeric sizes of IgA, and was associated with secretory component. The concentrations of IgA in tear samples were about 0.3% of those in matched serum samples. Thus, a plasma contribution is made to the IgA in tears, but greater than 99% of the tear IgA is synthesized locally in the lachrymal gland.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dahlgren U., Ahlstedt S., Hedman L., Wadsworth C., Hanson L. A. Dimeric IgA in the rat is transferred from serum into bile but not into milk. Scand J Immunol. 1981 Jul;14(1):95–98. doi: 10.1111/j.1365-3083.1981.tb00188.x. [DOI] [PubMed] [Google Scholar]
- Dean C. J., Styles J. M., Gyure L. A., Peppard J., Hobbs S. M., Jackson E., Hall J. G. The production of hybridomas from the gut associated lymphoid tissue of tumour bearing rats. I. Mesenteric nodes as a source of IgG producing cells. Clin Exp Immunol. 1984 Aug;57(2):358–364. [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M., McGee D. W., Shepard K. F. Lacrimal gland-directed B cell responses. J Immunol. 1985 Jul;135(1):95–99. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey J. F., Mitchell C. S., McKenzie S. J. The origins of secretory IgA in milk: a shift during lactation from a serum origin to local synthesis in the mammary gland. Ann N Y Acad Sci. 1983 Jun 30;409:452–460. doi: 10.1111/j.1749-6632.1983.tb26889.x. [DOI] [PubMed] [Google Scholar]
- Koertge T. E., Butler J. E. Dimeric M315 is transported into mouse and rat milk in a degraded form. Mol Immunol. 1986 Aug;23(8):839–845. doi: 10.1016/0161-5890(86)90069-6. [DOI] [PubMed] [Google Scholar]
- Kubagawa H., Bertoli L. F., Barton J. C., Koopman W. J., Mestecky J., Cooper M. D. Analysis of paraprotein transport into the saliva by using anti-idiotype antibodies. J Immunol. 1987 Jan 15;138(2):435–439. [PubMed] [Google Scholar]
- Lemaître-Coelho I., Yamakido M., Montgomery P. C., Langendries A. E., Vaerman J. P. Selective excretion of IgA in rat bronchial secretions: lack of significant contribution from plasma IgA. Immunol Commun. 1982;11(6):441–453. doi: 10.3109/08820138209050741. [DOI] [PubMed] [Google Scholar]
- Maekawa S., Hirano T., Kitagawa H., Ovary Z. Studies on immunity in hybridoma-bearing mice. B. Immunity against the hybridoma. I. Studies on the immune state of mice after rejection of the hybridoma. Int Arch Allergy Appl Immunol. 1985;76(2):174–181. doi: 10.1159/000233686. [DOI] [PubMed] [Google Scholar]
- Montgomery P. C., Ayyildiz A., Lemaitre-Coelho I. M., Vaerman J. P., Rockey J. H. Induction and expression of antibodies in secretions: the ocular immune system. Ann N Y Acad Sci. 1983 Jun 30;409:428–440. doi: 10.1111/j.1749-6632.1983.tb26887.x. [DOI] [PubMed] [Google Scholar]
- Montgomery P. C., Khaleel S. A., Goudswaard J., Virella G. Selective transport of an oligomeric IgA into canine saliva. Immunol Commun. 1977;6(6):633–642. doi: 10.3109/08820137709093472. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Deitcher D. L. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986 Aug 15;46(4):613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Shaw L. J., Fitzharris B., Peppard J., Hamilton M. J., Simpson M. T., Hunt T. M., Hinton R. H. Sources of proteins in human bile. Gut. 1985 May;26(5):500–509. doi: 10.1136/gut.26.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlans E., Peppard J. V., Payne A. W., Fitzharris B. M., Mullock B. M., Hinton R. H., Hall J. G. Comparative aspects of the hepatobiliary transport of IgA. Ann N Y Acad Sci. 1983 Jun 30;409:411–427. doi: 10.1111/j.1749-6632.1983.tb26886.x. [DOI] [PubMed] [Google Scholar]
- Orlans E., Peppard J., Fry J. F., Hinton R. H., Mullock B. M. Secretory component as the receptor for polymeric IgA on rat hepatocytes. J Exp Med. 1979 Dec 1;150(6):1577–1581. doi: 10.1084/jem.150.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppard J. V., Hobbs S. M., Jackson L. E., Rose M. E., Mockett A. P. Biochemical characterization of chicken secretory component. Eur J Immunol. 1986 Mar;16(3):225–229. doi: 10.1002/eji.1830160303. [DOI] [PubMed] [Google Scholar]
- Peppard J. V., Jackson L. E., Hall J. G., Robertson D. The transfer of immune complexes from the lumen of the small intestine to the bloodstream in sucking rats. Immunology. 1984 Oct;53(2):385–393. [PMC free article] [PubMed] [Google Scholar]
- Peppard J. V. Quantitative estimation of IgA in rats: a comparison of two methods. J Immunol Methods. 1979;31(1-2):129–139. doi: 10.1016/0022-1759(79)90293-x. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Peppard J. V., Hobbs S. M. Coccidiosis: characterization of antibody responses to infection with Eimeria nieschulzi. Parasite Immunol. 1984 Jan;6(1):1–12. doi: 10.1111/j.1365-3024.1984.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Scicchitano R., Husband A. J., Cripps A. W. Immunoglobulin-containing cells and the origin of immunoglobulins in the respiratory tract of sheep. Immunology. 1984 Jul;52(3):529–537. [PMC free article] [PubMed] [Google Scholar]
- Scicchitano R., Sheldrake R. F., Husband A. J. Origin of immunoglobulins in respiratory tract secretion and saliva of sheep. Immunology. 1986 Jun;58(2):315–321. [PMC free article] [PubMed] [Google Scholar]
- Shabo A. L., Kenyon K. R., Franklin R. M. Electron microscopic localization of a blood-tear barrier to tracer protein in the primate lacrimal gland. Lab Invest. 1973 Feb;28(2):185–193. [PubMed] [Google Scholar]
- Sheldrake R. F., Husband A. J., Watson D. L., Cripps A. W. Selective transport of serum-derived IgA into mucosal secretions. J Immunol. 1984 Jan;132(1):363–368. [PubMed] [Google Scholar]
- South M. A., Cooper M. D., Wollheim F. A., Hong R., Good R. A. The IgA system. I. Studies of the transport and immunochemistry of IgA in the saliva. J Exp Med. 1966 Apr 1;123(4):615–627. doi: 10.1084/jem.123.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. A., Allansmith M. R. Source of IgA in tears of rats. Immunology. 1984 Dec;53(4):791–799. [PMC free article] [PubMed] [Google Scholar]
- Virella G., Montgomery P. C., Lemaitre-Coelho I. M. Transport of oligomeric IgA of systemic origin into external secretions. Adv Exp Med Biol. 1978;107:241–251. doi: 10.1007/978-1-4684-3369-2_29. [DOI] [PubMed] [Google Scholar]


