Abstract
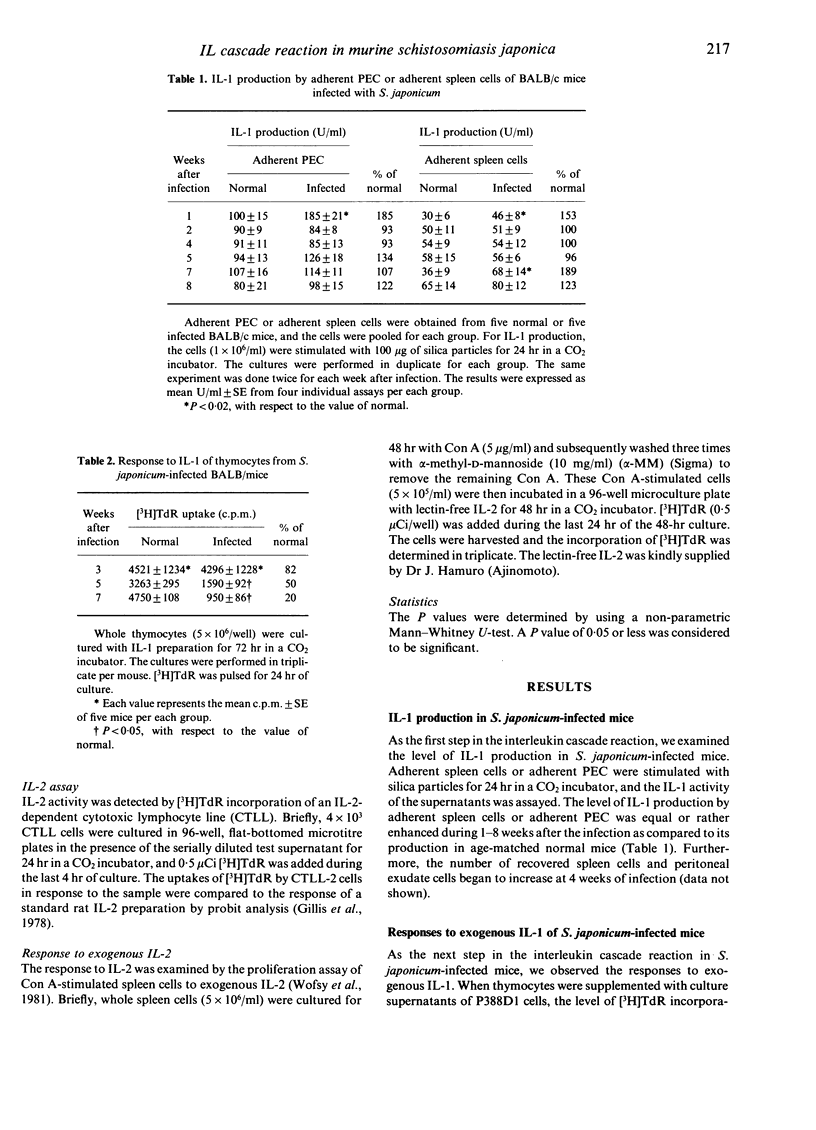
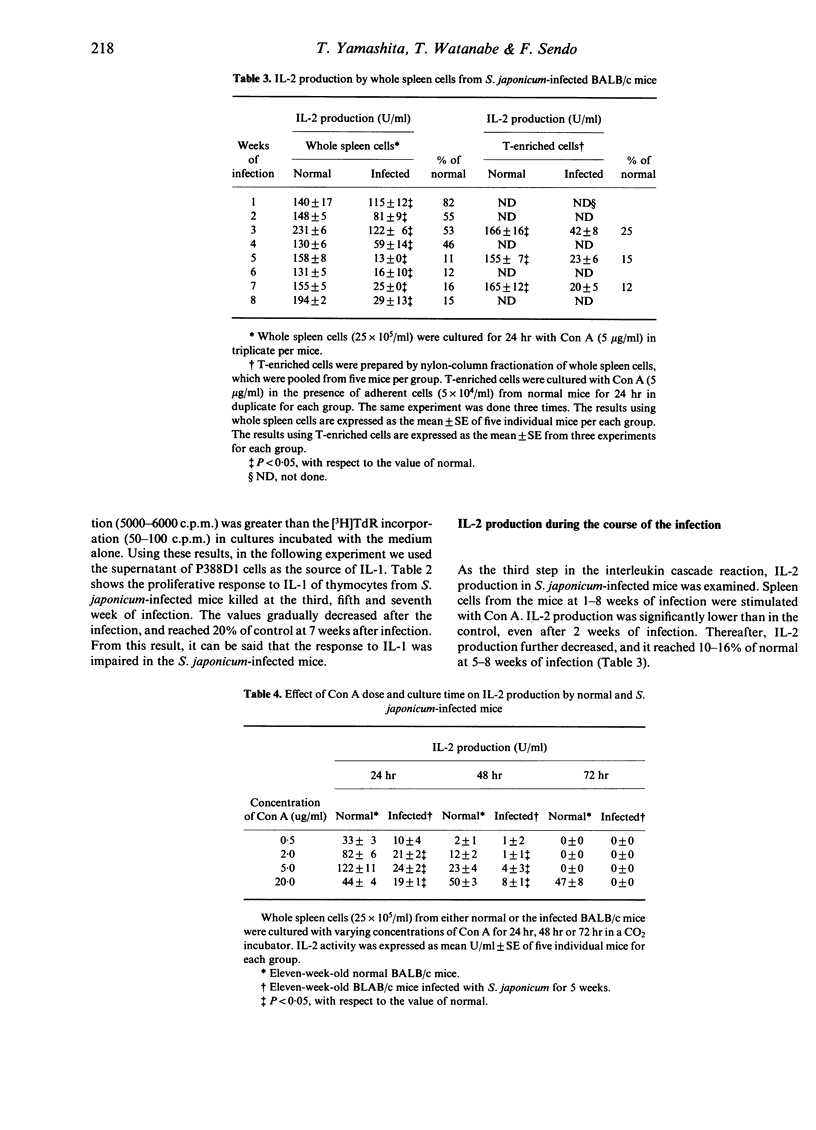
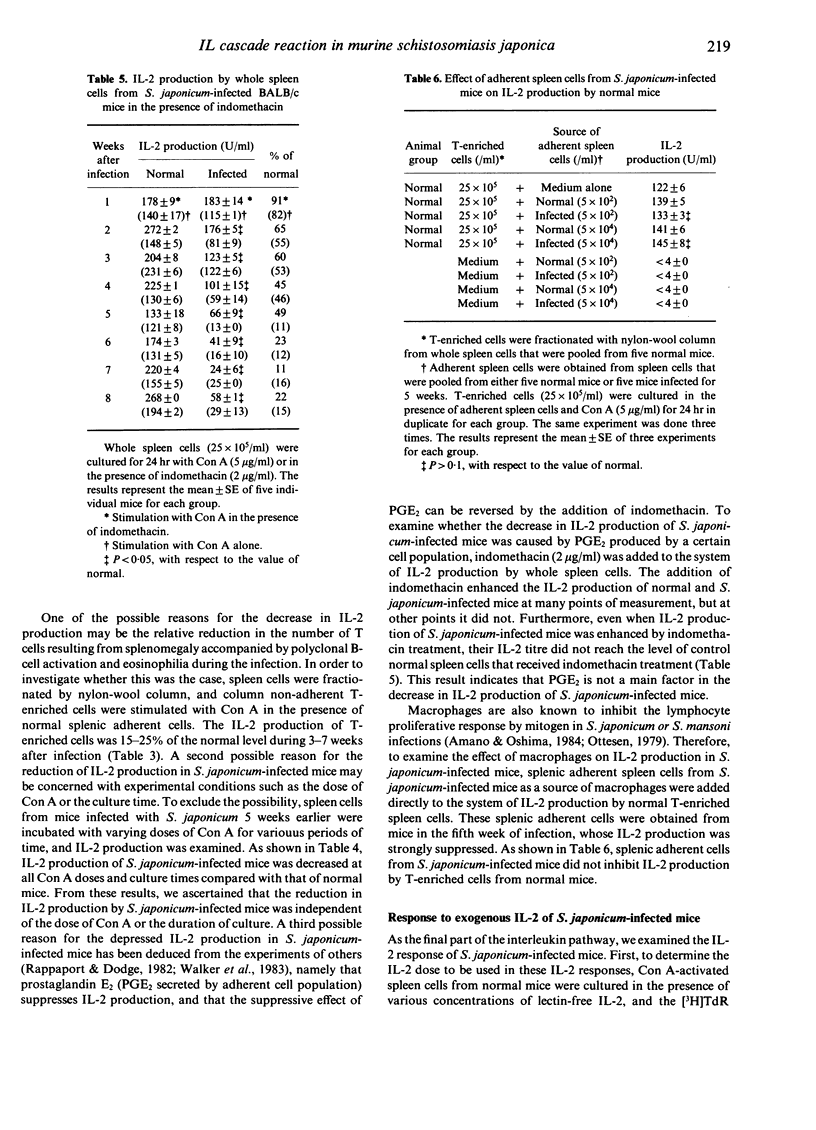
We examined the mechanisms of disturbed T-lymphocyte function that occurred during Schistosoma japonicum infection of BALB/c mice from the viewpoint of the interleukin cascade reaction. Each point of the interleukin cascade reaction was examined. First, IL-1 production by adherent peritoneal or spleen cells, as a source of macrophages, was normal or rather enhanced during the infection, the values being 100-180% of the control. Secondly, proliferative response to exogenous IL-1 of thymocytes from S. japonicum-infected mice progressively decreased during 3-7 weeks of infection. Thirdly, IL-2 production of S. japonicum-infected mice was significantly inhibited, even at 2 weeks of infection, and the activity was 10-20% of the control at 5-8 weeks of infection. Diminished IL-2 production was not caused by suppressive factors, such as PGE2 or suppressor macrophages, or a decrease in the number of IL-2 producing T cells. Finally, the response to exogenous IL-2 in S. japonicum-infected mice was suppressed markedly by 4 weeks of infection, and the responsiveness was reduced to 20% of the control at 8 weeks of infection. The mechanisms of disturbances in T-cell functions in S. japonicum-infected mice are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrus L., Prowse S. J., Lafferty K. J. Interleukin 2 production by both Ly2+ and Ly2- T-cell subsets. Scand J Immunol. 1981;13(3):297–301. doi: 10.1111/j.1365-3083.1981.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Chatenoud L., Klatzmann D., Fradelizi D. The mechanisms of inhibition of human IL 2 production. II. PGE2 induction of suppressor T lymphocytes. J Immunol. 1984 Apr;132(4):1851–1857. [PubMed] [Google Scholar]
- Conlon P. J., Henney C. S., Gillis S. Cytokine-dependent thymocyte responses: characterization of IL 1 and IL 2 target subpopulations and mechanism of action. J Immunol. 1982 Feb;128(2):797–801. [PubMed] [Google Scholar]
- Garb K. S., Stavitsky A. B., Mahmoud A. A. Dynamics of antigen and mitogen-induced responses in murine schistosomiasis japonica: in vitro comparison between hepatic granulomas and splenic cells. J Immunol. 1981 Jul;127(1):115–120. [PubMed] [Google Scholar]
- Garb K. S., Stavitsky A. B., Olds G. R., Tracy J. W., Mahmoud A. A. Immune regulation in murine schistosomiasis japonica: inhibition of in vitro antigen- and mitogen-induced cellular responses by splenocyte culture supernatants and by purified fractions from serum of chronically infected mice. J Immunol. 1982 Dec;129(6):2752–2758. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kawabata M., Hosaka Y., Kumada M., Matsui N., Kobayakawa T. Thymocytotoxic autoantibodies found in mice infected with Schistosoma japonicum. Infect Immun. 1981 May;32(2):438–442. doi: 10.1128/iai.32.2.438-442.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson E. L., Coutinho A. The role of mitogenic lectins in T-cell triggering. Nature. 1979 Jul 19;280(5719):239–241. doi: 10.1038/280239a0. [DOI] [PubMed] [Google Scholar]
- Lewert R. M., Yogore M. G., Jr, Blas B. L. Lymphocyte responsiveness to phytohemagglutinin and to worm and egg antigens in human schistosomiasis japonica. Am J Trop Med Hyg. 1979 Jan;28(1):92–98. doi: 10.4269/ajtmh.1979.28.92. [DOI] [PubMed] [Google Scholar]
- Ohta N., Minai M., Sasazuki T. Antigen-specific suppressor T lymphocytes (Leu-2a+3a-) in human schistosomiasis japonica. J Immunol. 1983 Nov;131(5):2524–2528. [PubMed] [Google Scholar]
- Ottesen E. A. Modulation of the host response in human schistosomiasis. I. Adherent suppressor cells that inhibit lymphocyte proliferative responses to parasite antigens. J Immunol. 1979 Oct;123(4):1639–1644. [PubMed] [Google Scholar]
- Rappaport R. S., Dodge G. R. Prostaglandin E inhibits the production of human interleukin 2. J Exp Med. 1982 Mar 1;155(3):943–948. doi: 10.1084/jem.155.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner Y., Linker-Israeli M., Sharon N. Separation of mouse thymocytes into two subpopulations by the use of peanut agglutinin. Cell Immunol. 1976 Jul;25(1):129–134. doi: 10.1016/0008-8749(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Wagner H., Hardt C., Heeg K., Pfizenmaier K., Solbach W., Bartlett R., Stockinger H., Röllinghoff M. T-T cell interactions during cytotoxic T lymphocyte (CTL) responses: T cell derived helper factor (Interleukin 2) as a probe to analyze CTL responsiveness and thymic maturation of CTL progenitors. Immunol Rev. 1980;51:215–255. doi: 10.1111/j.1600-065x.1980.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Walker C., Kristensen F., Bettens F., deWeck A. L. Lymphokine regulation of activated (G1) lymphocytes. I. Prostaglandin E2-induced inhibition of interleukin 2 production. J Immunol. 1983 Apr;130(4):1770–1773. [PubMed] [Google Scholar]
- Wofsy D., Murphy E. D., Roths J. B., Dauphinée M. J., Kipper S. B., Talal N. Deficient interleukin 2 activity in MRL/Mp and C57BL/6J mice bearing the lpr gene. J Exp Med. 1981 Nov 1;154(5):1671–1680. doi: 10.1084/jem.154.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J., Stadecker M. J., Dinarello C. A., O'Dea J. F. Fibroblast stimulation in schistosomiasis. V. Egg granuloma macrophages spontaneously secrete a fibroblast-stimulating factor. J Immunol. 1984 Jun;132(6):3142–3148. [PubMed] [Google Scholar]


