Abstract
The Lyme disease spirochete, Borrelia burgdorferi, inhabits the gut lumen of the tick vector. At this location the spirochete is exposed to host blood when a tick feeds. We report here on studies that were done with normal and complement-deficient (C3-knockout) mice to determine if the host complement system killed spirochetes within the vector. We found that spirochete numbers within feeding nymphs were not influenced by complement, most likely because host complement was inactivated within the vector. The Lyme disease outer surface protein A (OspA) vaccine is a transmission-blocking vaccine that targets spirochetes in the vector. In experiments with mice hyperimmunized with OspA, complement was not required to kill spirochetes within nymphs and to block transmission from nymphs to the vaccinated host. However, host complement did enhance the ability of OspA antibody to block larvae from acquiring spirochetes. Thus, the effects of OspA antibody on nymphal transmission and larval acquisition appear to be based on different mechanisms.
Lyme disease spirochetes (Borrelia burgdorferi) are extracellular bacteria that cause systemic infections in vertebrate hosts. In the tick vector, infections are mainly confined to the lumen of the gut (27). At this location the bacteria are exposed to host blood whenever a tick feeds. Here we report on studies to determine if spirochetes within the tick are sensitive to host complement proteins in the blood meal.
B. burgdorferi grown in culture activates complement even in the absence of specific antibodies against Borrelia, and complement protein C3b binds to the bacterial surface (3, 4, 21, 34). The B. burgdorferi ERP (OspEF-related protein) family of outer membrane proteins binds to factor H and factor H-like protein 1 (FHL-1), which are host serum proteins that inactivate C3b (14, 15, 30). Thus, spirochetes appear to resist the bactericidal effects of activated complement by binding to host proteins that inactivate C3b. Many ERPs are expressed in the mammalian host and are likely to play a role in protecting B. burgdorferi from complement in the mammal (30). It is not known if spirochetes utilize special mechanisms for protecting themselves from complement in the tick blood meal.
Active host complement in the tick blood meal could have a profound impact on B. burgdorferi within ticks. Complement in the blood meal may reduce the number of spirochetes within the tick gut, and this, in turn, may lower the number of infected ticks as well as the ability of infected ticks to transmit spirochetes. Thus, spirochetes within the tick may have evolved specific mechanisms such as the expression of particular ERPs to escape from complement in the blood meal (30). It is also possible that complement may be inactivated by factors produced by the feeding tick and that spirochetes may not require special mechanisms for protecting themselves from host complement in the tick gut (25, 33, 36).
Studies of the fate of host complement in the tick may also reveal new information about the OspA Lyme disease vaccine. The OspA protein is primarily expressed by spirochetes in the tick gut, where it functions as a receptor for anchoring spirochetes to the gut epithelium (20). The OspA vaccine selectively targets spirochetes expressing OspA in the tick gut and blocks transmission from the tick to the host (9). OspA antibodies also prevent uninfected larval ticks from acquiring an infection from infected mice by targeting spirochetes as they enter the tick and upregulate the expression of OspA (8). It is not known if active complement in the blood meal is required for the effects of OspA antibody on Borrelia within ticks.
Here we describe studies with normal mice and transgenic mice without a functional complement system to understand the influence of host complement on B. burgdorferi within feeding ticks. The studies were done both in the absence and in the presence of specific antibody against B. burgdorferi.
MATERIALS AND METHODS
B. burgdorferi and culture conditions.
A clonal population of low-passage-number Westchester strain B. burgdorferi sensu stricto grown in Barbour-Stoenner-Kelly II medium at 33°C to mid-log density (1 × 107 to 3 × 107 cells/ml) was used in this study.
Mice and ticks.
Transgenic C57BL/6 mice deficient in component C3 of the complement system (kindly provided by M. C. Carroll, Center for Animal Resources and Comparative Medicine, Harvard Medical School, Boston, Mass.) and wild-type (WT) C57BL/6 mice were bred and used for the experiments (35). The mice were caged individually and provided with antibiotic-free food and water ad libitum.
Ixodes scapularis ticks infected with the Westchester strain of B. burgdorferi were raised as described previously (23) and used for various experiments in this study.
Estimating spirochete number within nymphs.
Westchester strain-infected nymphal ticks were allowed to feed on complement-deficient (CD) and complement-sufficient mice. Nymphs were then removed with fine forceps at 48, 60, and 72 h into the blood meal. The spirochete load within individual nymphs that had not fed (0-h point) or fed for 48, 60, and 72 h was assessed by immunofluorescence staining of individual tick homogenates as described below. Four or five nymphs were used for each time point. Nymphs were homogenized individually in 40 μl of phosphate-buffered saline (PBS) in 0.5-ml Eppendorf tubes with disposable pestles (Nalgene Nunc International, Naperville, Ill.). Five microliters of the homogenates was spotted on silylated slides (PGC Scientifics, Frederick, Md.). The spots were air dried, acetone fixed, blocked with 5% fetal calf serum in PBS (pH 7.2), and incubated with fluorescein isothiocyanate (FITC)-conjugated anti-Borrelia antibodies for 1 h at room temperature. Following incubation, the slides were washed three times with PBS, air dried, and mounted with Aqua-Polymount (Polysciences Inc., Warrington, Pa.). The number of spirochetes in 10 fields (400× magnification) was counted, and this number was used to estimate the mean number of spirochetes per single 400× microscope field. The total count in 10 fields ranged from a low of 5 spirochetes (0-h point) to a high of 1,404 spirochetes (72-h point). A 5-μl spot on the slide has an area equivalent to 49 400× microscope fields. The number of spirochetes in each nymph was calculated by the following equation: (mean number of bacteria in single 400× field) × (49 fields) × (40 μl/5 μl).
Immunization and challenge experiments.
Groups of CD and complement-sufficient mice were injected subcutaneously with 20 μg of OspA glutathione S-transferase (GST) fusion protein in complete Freund's adjuvant. Control mice received similar amounts of GST. Mice were boosted with 10 μg of OspA-GST in incomplete Freund's adjuvant twice at 2-week intervals after the first dose. Two weeks after the final boost the mice were bled to measure OspA antibody titer and then challenged with five to seven infected nymphs per mouse. Mice were killed 3 weeks after the nymphs had fallen off and tested for Borrelia infection by serology (Western blotting) and organ culture in Barbour-Stoenner-Kelly II medium. Infected nymphal ticks that had fed to repletion on the OspA-GST- and GST-immunized mice were homogenized individually in PBS, and the spirochete load in the nymphs was assessed by immunofluorescence staining as described above.
Acquisition of spirochetes by larval ticks.
CD and complement-sufficient mice were infected with the Westchester strain of B. burgdorferi by infected nymphal tick feeding. Infection was confirmed by serology with Western blotting. Infected CD and complement-sufficient mice were passively immunized with hyperimmune OspA antibodies raised in CD mice as described above. Control mice were passively immunized with control serum raised against GST. Mice in the experimental and control groups received 100 μl of hyperimmune serum subcutaneously and 100 μl intraperitoneally. Twenty-four hours after passive immunization, the mice were anesthetized with ketamine-xylazine, and approximately 100 larvae were placed on each mouse. The larvae that had fed to repletion were collected and stored in groups of 20 in a humid chamber. Fifteen larvae from each mouse were tested individually for spirochetes by immunofluorescence 2 weeks after the blood meal.
In vitro complement assays.
Sera for bactericidal assays were collected from normal (normal mouse serum [NMS]) and CD mice (CD mouse serum [CDS]) into red-top Vacutainer tubes. The blood was allowed to clot at 37°C for 30 min. Subsequently, serum was separated by centrifuging the clotted blood at 4°C, clarified by passage through 0.45-μm-pore-size syringe filters (Millipore), and stored in aliquots at −70°C. A pool of sera from six mice was used for the assays. Nymphal tick gut contents (TGC) for complement assays were collected by allowing uninfected nymphs to feed on CD and complement-sufficient mice. At 72 h into the blood meal the nymphs were removed with fine forceps. The nymph's surface was disinfected with 70% ethanol for 5 min. The exoskeleton was punctured with a 27-gauge needle, and the gut contents were collected at 4°C as follows. The nymph was gently pressed, and gut content was collected through the puncture site into gelatin vernol buffer (GVB) with 2 mM phenylmethylsulfonyl fluoride at 4°C. Contents from two nymphs were collected into 10 μl of GVB and frozen immediately at −80°C.
The serum bactericidal assays were performed as described previously with slight modifications (13). Spirochetes (Westchester strain) from culture or purified from feeding nymphs (24) were centrifuged at 2,040 × g for 15 min at room temperature, and the cell pellet was washed once with minimal essential medium (MEM; GIBCO BRL, Rockville, Md.). The cells were adjusted to the desired concentration in MEM. In each assay 1.2 × 106 spirochetes were used in a final volume of 100 μl. In each experiment spirochetes (cultured or tick derived) were resuspended in MEM alone as well as with different sources of specific antibody and complement. A hyperimmune Borrelia antiserum raised in CD mice was used as a source of specific antibody against spirochetes. Initially the bacteria were incubated with the hyperimmune serum (1:4,000 dilution) at 37°C for 15 min. Next, the following sources of serum were added to different tubes: NMS, heat-inactivated NMS (56°C for 30 min), CDS, gut contents from nymphs fed on WT mice (WT TGC), and gut contents of nymphs fed on CD mice (CD TGC). All the serum sources were added to a final concentration of 10%. When equal volumes of mouse serum and TGC were probed for C3 on a Western blot, the NMS sample yielded a signal that was approximately four times more intense than the WT TGC (see Fig. 2). Thus, in the in vitro assays we used four times more TGC (40 μl) than NMS (10 μl) to ensure that we had similar levels of serum protein in each assay. The reaction mixtures were incubated for 2 h at 37C in a 2% CO2 atmosphere. At the end of the incubation period the spirochetes were stained with the Live/Dead stain (Molecular Probes, Eugene, Oreg.) following the manufacturer's instructions. The number of live spirochetes per microscope field was estimated by counting 10 fields and then determining the average number of live bacteria per field. The proportion of bacteria killed in each assay was determined by setting the number of spirochetes in MEM alone as 100%.
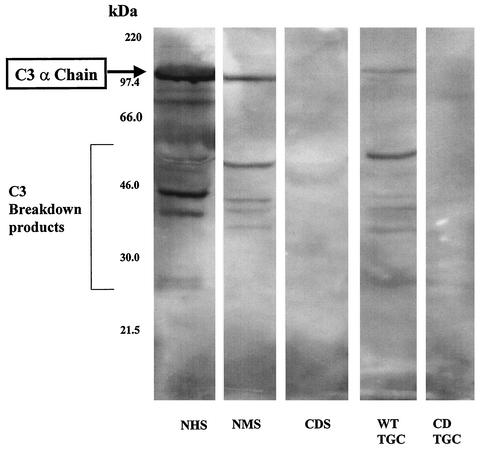
FIG. 2.
Detection of C3 in serum and the gut contents of partially fed nymphs. Protein extracts were prepared from normal human serum (NHS), NMS, CDS, WT TGC, and CD TGC and analyzed by Western blotting for the presence of C3 protein.
Detection of C3 in serum and nymphal TGC.
The proteins in NMS (0.2 μl), CDS (0.2 μl), CD TGC (4 μl), and WT TGC (4 μl) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The resolved proteins were transferred onto polyvinylidene difluoride membranes and probed with a protein detector Western blot kit (Kirkegaard & Perry Laboratories, Gaithersburg, Md.). The membrane was blocked with the blocking solution provided in the kit and probed with polyclonal goat anti-C3 antibodies (Sigma, St. Louis, Mo.) that cross-react with human and mouse C3 components at a 1:400 dilution in the blocking solution. The secondary antibody was a horseradish peroxidase-conjugated anti-goat immunoglobulin used at a 1:1,000 dilution. Antibody reactivity was detected by the chemiluminescence method with the LumiGlo substrate provided in the kit.
Assay for C3 deposition on spirochetes.
Westchester strain-infected nymphs were allowed to feed on naïve complement-sufficient and CD mice for 48 h. Following feeding, the nymphs were removed with fine forceps and homogenized in 40 μl of GVB with 2 mM phenylmethylsulfonyl fluoride. In other experiments 109 Westchester strain spirochetes grown in culture were incubated with different serum sources as described above, pelleted, washed twice with GVB, and resuspended in 1 ml of GVB. Five microliters of the suspended spirochetes (from nymphs or culture) was spotted onto silylated slides (PGC Scientifics), air dried, and acetone fixed. The spots were blocked with 5% fetal calf serum in PBS and incubated with polyclonal goat anti-C3 antibodies (Sigma Chemical Co.) for 1 h at room temperature. Following incubation, the slides were washed three times with PBS and incubated with FITC-conjugated anti-goat antibodies. The slides were then washed three times with PBS, air dried, mounted with Aqua-Polymount, and viewed with a fluorescence microscope.
RESULTS
Spirochete number within nymphs feeding on WT and CD mice.
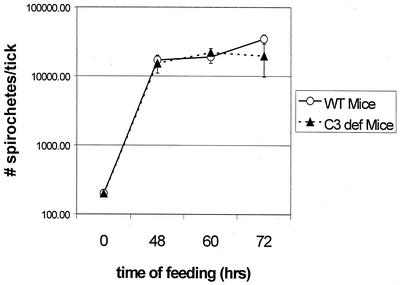
Spirochetes are mainly confined to the lumen of the gut of infected nymphal ticks. When a nymph feeds on a naïve mouse, the spirochetes multiply and increase in number. Given that cultured Borrelia organisms activate complement even in the absence of specific antibody, it is possible that the final number of bacteria within a feeding nymph is a result of spirochete multiplication as well as killing by complement in the incoming blood meal. We carried out experiments to investigate the effect of host complement on the load of spirochetes within feeding nymphs. Nymphal ticks infected with B. burgdorferi were allowed to feed on naïve complement-sufficient and CD mice for various time periods, and the spirochete number inside each nymph was determined by immunofluorescence. Spirochetes multiplied to equal numbers within the nymphs independently of the presence or absence of a functional complement system in the vertebrate host (Fig. 1). Thus, host complement did not suppress spirochete growth in feeding nymphal ticks.
FIG. 1.
Growth of B. burgdorferi within nymphs feeding on WT mice and CD mice. Homogenates were prepared from nymphs that had fed for different time periods and stained with an FITC-conjugated anti-Borrelia antibody to determine the mean number of spirochetes within each infected nymph as described in Materials and Methods. The data at each time point are based on four or five individual infected nymphs, and the errors bars represent the standard deviations.
The role of host complement in OspA-mediated transmission-blocking immunity.
The studies described above proved that in the absence of specific Borrelia antibody spirochete growth within the feeding nymph was not sensitive to complement. We next set out to investigate if host complement was capable of killing spirochetes if the incoming blood meal contained specific antibodies against spirochetal antigens expressed in the nymphal tick gut. When animals vaccinated with B. burgdorferi OspA are challenged with infected nymphs, OspA antibodies in the incoming blood meal kill bacteria in the nymphal tick gut and also block the movement of spirochetes from the nymph to the host (9, 11). It is unclear if host complement is absolutely required for the bactericidal effects of OspA antibody within the nymphal tick. We performed experiments with groups of WT and CD mice immunized with recombinant OspA (OspA-GST) and a control antigen (GST). Spirochetes were killed within nymphs that fed on OspA-immunized mice with and without a functional complement system (Table 1). Furthermore, OspA antibodies blocked transmission to both WT and CD mice (Table 1). Thus, in hyperimmunized mice containing high levels of OspA antibody an intact complement system in the host was not required to kill spirochetes within feeding nymphs and block transmission to mice.
TABLE 1.
Ability of OspA vaccination to block the transmission of B. burgdorferi from nymphs to mice in the absence of complementa
| Vaccine antigen | Result for mouse group:
|
|||
|---|---|---|---|---|
| WT
|
CD
|
|||
| Tick infection (nymph)b | Mouse infectionc | Tick infection (nymph)b | Mouse infectionc | |
| GST | + | 4/4 | + | 5/5 |
| OspA-GST | − | 0/4 | − | 0/5 |
Mice deficient and sufficient in complement were immunized with OspA-GST or GST alone and challenged with infected nymphal ticks.
To assess the survival of spirochetes within the ticks that fed on immunized mice, homogenates of the ticks were stained with anti-Borrelia FITC-conjugated antibodies and counted under immunofluorescence. No spirochetes were observed in the ticks that fed on OspA-GST-immunized mice, while the ticks that fed on the GST-immunized mice were all found to be positive for the spirochetes.
Results are number of mice infected/total number of mice.
Acquisition of B. burgdorferi by larval ticks.
The life cycle of the Lyme disease spirochete involves larval ticks acquiring spirochetes from infected mice, the infected larvae molting to the nymphal stage, and infected nymphal ticks transmitting spirochetes to naïve mice. Having observed no role for complement within infected nymphal ticks, we wanted to determine if host complement had an effect on the acquisition of spirochetes by larval ticks. Larvae become infected as early as 24 h after feeding on an infected mouse (8). When a mouse with an established B. burgdorferi infection is actively or passively immunized with OspA antibody, the infection in the mouse is not cleared (10). However, OspA antibodies do inhibit the movement of spirochetes from the infected mouse to feeding larval ticks (5, 8). Experiments were done to determine if host complement had an effect on larval acquisition of spirochetes in the presence and absence of OspA antibody.
CD and complement-sufficient mice were infected by placing infected nymphs on the mice. Two weeks later, infection was confirmed in all mice by serology. A polyclonal OspA antibody (prepared in CD mice) or PBS was passively administered to the infected mice, and 24 h later uninfected larval ticks were placed on all the mice and allowed to feed to repletion. When larvae recovered from WT and CD mice treated with PBS were compared, no differences were observed in the number of infected larvae or in the load of spirochetes within each infected larva (Table 2). These results demonstrate that, in the absence of specific antibody, host complement had no effect on larval acquisition of spirochetes. When larvae recovered from mice treated with OspA antibody were compared, a clear difference was observed between larvae that fed on WT mice and those that fed on CD mice (Table 2). Larval infection was more efficiently blocked by OspA antibody when the larvae fed on mice with an intact complement system than when the larvae fed on mice with no functional complement. These data indicate that host complement does play a role in the OspA antibody-mediated block in larval acquisition of spirochetes.
TABLE 2.
Differences in the abilities of OspA antibody to block the acquisition of B. burgdorferi by larval ticks in the presence and absence of complement in the hosta
| Larva group | No. of larvae tested | No. of infected larvae (degree of infectionb) |
|---|---|---|
| Fed on WT mouse | 15 | 11 (4 high, 3 moderate, 4 low, 4 uninfected) |
| Fed on CD mouse | 15 | 10 (2 high, 3 moderate, 5 low, 5 uninfected) |
| Study I | ||
| Fed on WT mouse + Abc | 15 | 4 (4 low, 11 uninfected) |
| Fed on CD mouse + Ab | 15 | 8 (1 high, 5 moderate, 2 low, 7 uninfected) |
| Study II | ||
| Fed on WT mouse + Ab | 15 | 6 (2 high, 3 moderate, 1 low, 9 uninfected) |
| Fed on CD mouse + Ab | 15 | 12 (4 high, 4 moderate, 4 low, 3 uninfected) |
Larval ticks were allowed to feed to repletion on B. burgdorferi-infected WT or CD mice that were untreated or passively administered OspA antibody. Ten days after feeding, the larvae were homogenized individually on slides and stained with FITC-conjugated anti-Borrelia sp. antibodies. The results from two independent passive OspA antibody transfer experiments are presented.
Infection of the positive samples was graded based on the average number of spirochetes per microscope field: 1 to 10, low; 11 to 50, moderate; more than 50, high.
Ab, antibody.
Is host complement active in the nymphal tick gut?
From the experiments with infected nymphal ticks, it was clear that host complement did not have an effect on spirochetes within nymphs. Borrelia in the nymphal gut may express genes that confer a high level of resistance to complement. Alternatively, spirochetes within the nymph may have been protected from complement because complement in the blood meal is in an inactive state. We performed experiments to determine if spirochetes within feeding nymphs were more complement resistant than were bacteria grown in culture. For this purpose we used a procedure recently developed in our laboratory to purify B. burgdorferi from feeding nymphs (24). Similar numbers of spirochetes (3 × 105 to 5 × 105) grown in culture or purified from feeding nymphs were incubated with 10% NMS for 2 h at 37°C in a CO2 atmosphere. Under these conditions 42% of the cultured bacteria and 95% of the bacteria purified from nymphs were killed. Thus, bacteria within the nymph were not especially resistant to serum-mediated killing; if at all, they appeared to be more sensitive to mouse serum than were spirochetes grown in culture.
Next, we performed experiments to test if host complement in the blood meal was active within the nymph. Initially experiments were done to determine if intact host C3 was present in the blood meal. Nymphs were placed on WT and CD mice. Protein extracts were prepared from NMS and CDS as well as from nymphs that had partially fed (72 h) on WT and CD mice. The extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with anti-C3 antibodies in a Western blot assay. As expected full-length and cleaved C3 were observed in NMS but not in CDS (Fig. 2). Full-length C3 and cleaved C3 fractions were also observed in extracts from nymphs that fed on WT mice (Fig. 2), indicating that intact C3 was present within the nymph.
Borreliacidal assay for detection of complement activity in the nymphal tick gut.
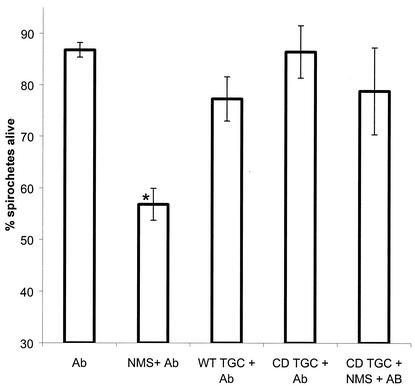
Having confirmed the presence of intact C3 protein in the nymphal tick gut, we performed experiments to detect complement activity in the gut. Activity was measured by an in vitro borreliacidal assay in which cultured spirochetes were incubated with potential complement sources and specific Borrelia antibody. When spirochetes were incubated with specific Borrelia antibody alone (no complement), approximately 13% of the spirochetes were killed (Fig. 3). When specific Borrelia antibody and a complement source (NMS) were added, approximately 50% of the spirochetes were killed (Fig. 3). Unlike NMS, gut contents from nymphs that had partially fed on mice did not enhance the ability of antibody to kill spirochetes, indicating that no active complement was present in the guts of nymphs (Fig. 3). This conclusion was further strengthened when nymphal gut extracts were found to have an inhibitory effect on the ability of NMS and specific antibody to kill spirochetes (Fig. 3). Thus, although C3 was present in the gut of partially fed nymphs, no complement activity was observed. Instead, nymphs appeared to have an activity that inhibited complement activity in NMS.
FIG. 3.
In vitro borreliacidal assay to detect active complement in nymphal tick gut extracts. Spirochetes from culture were incubated with specific anti-Borrelia antibody alone (Ab), NMS and specific antibody (NMS + Ab), gut contents from nymphs feeding on WT mice and specific antibody (WT TGC + Ab) or NMS, and gut contents from nymphs feeding on CD mice and specific antibody (CD TGC + NMS + Ab). The data are presented as the percentages of surviving spirochetes in comparison to a control not treated with antibody or complement source. The asterisk indicates that the NMS + Ab group had significantly fewer surviving spirochetes than did the other three groups (analysis of variance test followed by Tukey highest significant difference test, P < 0.05). The Ab-alone, WT TGC + Ab, and CD TGC + NMS + Ab groups were not significantly different from one another.
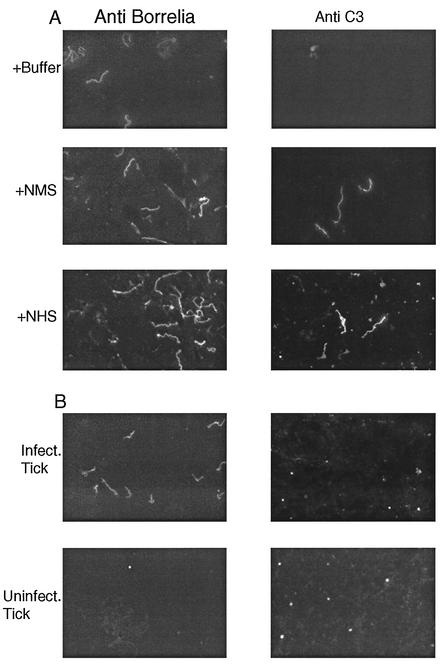
Our conclusion that host complement was not active in the nymphal gut was further supported by studies to detect the deposition of C3 on the surface of spirochetes. When cultured spirochetes were incubated with NMS or normal human serum, C3 was deposited on the surface of the bacteria (Fig. 4A). No C3 deposition was observed when cultured spirochetes were incubated with heat-inactivated NMS or TGC from partially fed ticks (data not shown). When homogenates from partially fed infected nymphs were stained with the C3 antibody, no spirochetes were stained, indicating that C3 in the blood meal was not deposited on the surface of the bacteria within the gut (Fig. 4B). Although we did not observe spirochete-shaped structures staining with the C3 antibody, we did observed bright punctate material, which is probably free C3, as it was also present in gut smears prepared from uninfected ticks (Fig. 4B). Thus, although C3 is present in the blood meal entering the gut (Fig. 2), we found no evidence that it was in a state that permitted deposition on the surface of spirochetes.
FIG. 4.
Indirect immunofluorescence assay to detect C3 deposition on the surface of B. burgdorferi. C3 in blood entering the vector was not deposited on the surface of spirochetes. (A) Cultured spirochetes were incubated with buffer alone, NMS, or normal human serum (NHS). The samples were fixed and stained with an anti-Borrelia polyclonal antibody or anti-C3 polyclonal antibody. Spirochetes were stained with the C3 antibody when they were exposed to NMS or NHS. (B) Infected and uninfected nymphs were allowed to partially engorge on a WT mouse, and the tick guts were homogenized and stained with an anti-Borrelia polyclonal antibody or anti-C3 polyclonal antibody. Spirochetes in the nymphal tick gut were not stained with the C3 antibody. The punctate staining observed with the C3 antibody in infected and uninfected nymphs probably represents free C3 in the blood meal.
DISCUSSION
The Lyme disease spirochete is exposed to host blood and components of the host's immune system in the gut of feeding ticks (28). The present study was undertaken to determine if spirochetes in the vector were sensitive to killing by host complement in the presence and absence of specific Borrelia antibody. To our surprise, host complement proteins in the blood meal did not influence spirochete numbers within infected nymphs.
B. burgdorferi alters the expression of genes at different stages in the vector and host, and it is plausible that the organisms in the tick gut may produce proteins that confer complement resistance. Recent studies by several groups have implicated the B. burgdorferi ERP family of surface proteins in complement resistance (1, 14, 15, 30). Some ERPs are expressed by spirochetes in the nymphal tick gut, and the presence of these proteins on the bacterial surface may explain our results (12). To test this idea, we purified B. burgdorferi bacteria from partially engorged nymphs and then exposed them to NMS. The bacteria purified from nymphs were serum sensitive and, in fact, appeared to be more sensitive to serum than were organisms grown in culture. Based on these results, it is unlikely that innate serum resistance of spirochetes in the vector accounts for our results.
Another potential explanation for our results is that host complement is inactive in the nymphal tick gut, and our data support this hypothesis. Although complement protein C3 was deposited on the surface of spirochetes incubated with NMS, we did not observe any C3 deposition on spirochetes incubated with the gut contents of partially fed nymphs. Furthermore, unlike NMS, gut contents from partially fed nymphs did not enhance the bactericidal effects of Borrelia antibody in an in vitro assay. In fact, nymphal TGC inhibited the bactericidal effects of complement in NMS.
Why might host complement not be active within the vector? The complement system involves the action of more than 23 host proteins, and it is possible that the environment within the nymph may not be conducive for the coordinated activation of these proteins. Alternatively, the nymph may actively produce proteins that inhibit complement activity to protect tick epithelial surfaces that come into contact with host blood as well as to suppress host immunity at the site of tick feeding (25). This hypothesis is supported by recent studies that have led to the identification of two novel proteins in tick saliva that inhibit host complement (7, 33). It is possible that these proteins secreted into the host are also taken up in the blood meal entering the nymphal tick gut, where they may protect tick tissue as well as pathogens harbored by the tick.
Others have also studied the fate of host complement within arthropod vectors, mainly mosquitoes (18, 32). Unlike in ticks, complement was found to be active in the mosquito gut, at least for the first several hours after the blood meal, and host complement was observed to reduce the number of malarial gametocytes infecting the mosquito. The observation that mosquitoes contain functional complement, unlike ticks, is not surprising given the different feeding behavior and physiology of these blood-feeding arthropods. Mosquitoes feed for a few minutes and probably do not have to inhibit the host's immune responses, including complement, to the same extent as do hard ticks that have to continuously feed on their host for many days (2). Furthermore, the mosquito blood meal is digested in the lumen of the gut, where the blood meal is enclosed in a peritrophic membrane that protects the gut epithelial lining from potentially harmful molecules in the blood meal (26). In contrast ticks digest their blood meal intracellularly by phagocytosing gut content, and it is unclear if hard ticks have a well-defined peritrophic matrix like mosquitoes do (31). The peritrophic membrane and secreted digestive enzymes are likely to protect mosquitoes' tissue from host complement, whereas the digestive strategy employed by ticks may require the production of specific molecules by the vector for inactivating host complement.
Our conclusion that host complement is inactive within the vector contradicts a recent model proposed to explain the host preference of different B. burgdorferi sensu lato species (16). B. valisiana, B. turdi, and certain strains of B. garinii are found in enzootic cycles with birds as the main vertebrate reservoir, whereas B. afzelii, B. bisseti, B. japonica, and most strains of B. garinii appear to use rodents as the main reservoir host. These host association patterns are also reflected in the complement sensitivity of these Borrelia species, i.e., bird-associated species are resistant to bird complement and sensitive to rodent complement, and the opposite is true for the rodent-associated species. Kurtenbach and colleagues (17) have proposed that sensitivity of spirochetes to host complement is a key determinant of the host preferences of species in the B. burgdorferi sensu lato complex. They also postulate that the tick gut is the main site at which complement-mediated selection occurs, because the spirochetes come into close contact with host blood in the vector. Our data indicating that host complement is not active within the vector indicate that complement-mediated selection is unlikely to occur within the vector. Rather, if complement sensitivity is responsible for the host association patterns of different Borrelia species, the selection is likely to occur once the spirochetes enter the host and not in the vector (17).
Although our data point to complement being inactivated within the nymphal tick gut, this hypothesis should be further tested because the assays used here are all indirect measures of complement activity within the gut. For example, the lack of C3 deposition on spirochetes may be due to specific tick molecules binding to the spirochetes and not inactivation of the complement cascade. Furthermore, the inhibitory activity in TGC observed in vitro may be derived from a compartment other than the lumen of nymphal tick gut and this activity may not be relevant to events occurring within the gut lumen. Attempts are under way to purify this activity to determine its structure and location of action within the tick.
B. burgdorferi OspA is the only Lyme disease vaccine that has consistently proven efficacious in animal and human studies (22). The OspA vaccine protects hosts by generating antibodies that enter the gut of the vector and block the movement of spirochetes from the vector to the host. The exact mechanism by which OspA antibodies block transmission is not known. In vitro the borreliacidal effects of OspA antibodies are greatly enhanced in the presence of complement (19, 29). However, our results do not support a role for complement in transmission-blocking immunity because spirochetes were killed within infected nymphs feeding on OspA-vaccinated C3-deficient mice. In these studies we used mice that had been hyperimmunized with OspA, and the high levels of OspA antibody may have masked a role for complement apparent at lower titers of antibody. However, in preliminary studies with low concentrations of OspA antibody we have observed no role for complement in transmission-blocking immunity (C. Gipson and A. de Silva, unpublished data).
Our conclusion that host complement is inactive in the vector also has implications for developing arthropod-specific transmission-blocking vaccines against Borrelia and other tick-borne pathogens. The development and evaluation of these vaccines should not be based on eliciting antibody responses that fix complement. Rather, the ability of antibodies to kill the pathogen of interest in the absence of complement or the ability of antibody to specifically interfere with interactions between the vector and pathogen should be given precedence.
During the natural transmission cycle of the Lyme disease spirochete, larval ticks acquire the infection and nymphs transmit the infection (6). In addition to the effects on nymphal transmission, OspA antibody also blocks larvae from acquiring infections from infected mice (8). Here we report that the effect of OspA antibody on larval acquisition was enhanced in the presence of a functional complement system in the host. Thus, the effects of OspA antibody on nymphal transmission and larval acquisition appear to be based on fundamentally different mechanisms, one independent of and the other dependent on host complement. Larval ticks and nymphal ticks may inactivate host complement to different extents. Alternately, the spirochetes en route from the host to larvae may produce OspA in the host, leading to opsonization and complement-mediated clearance in the host before the spirochetes ever get into the vector. Further studies are needed to understand the exact mechanisms by which antibody blocks the movement of spirochetes between vector and host.
Acknowledgments
This work was supported by Public Health Service grants KO1 AR02061 and RO1 AR47948 from the National Institute for Arthritis and Musculoskeletal and Skin Diseases and an Arthritis Investigator Award from the Arthritis Foundation.
We thank Chris Elkins (Department of Medicine, UNC-CH) and members of the de Silva laboratory, especially Clay Gipson, for their advice and help with these studies.
Editor: J. D. Clements
REFERENCES
- 1.Alitalo, A., T. Meri, L. Ramo, T. S. Jokiranta, T. Heikkila, I. J. Seppala, J. Oksi, M. Viljanen, and S. Meri. 2001. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect. Immun. 69:3685-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J. F. 2002. The natural history of ticks. Med. Clin. N. Am. 86:205-218. [DOI] [PubMed] [Google Scholar]
- 3.Brade, V., I. Kleber, and G. Acker. 1992. Differences of two Borrelia burgdorferi strains in complement activation and serum resistance. Immunobiology 185:453-465. [DOI] [PubMed] [Google Scholar]
- 4.Breitner-Ruddock, S., R. Wurzner, J. Schulze, and V. Brade. 1997. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med. Microbiol. Immunol. (Berlin) 185:253-260. [DOI] [PubMed] [Google Scholar]
- 5.Brunet, L. R., P. Katavolos, A. Spielman, and S. R. Telford III. 1997. Anti-OspA antibody reduces reservoir competence of mice for Borrelia burgdorferi, the agent of Lyme disease. Med. Vet. Entomol. 11:198-200. [DOI] [PubMed] [Google Scholar]
- 6.Burgdorfer, W. 1989. Vector/host relationships of the Lyme disease spirochete, Borrelia burgdorferi. Rheum. Dis. Clin. N. Am. 15:775-787. [PubMed] [Google Scholar]
- 7.Das, S., G. Banerjee, K. DePonte, N. Marcantonio, F. S. Kantor, and E. Fikrig. 2001. Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J. Infect. Dis. 184:1056-1064. [DOI] [PubMed] [Google Scholar]
- 8.de Silva, A. M., D. Fish, T. R. Burkot, Y. Zhang, and E. Fikrig. 1997. OspA antibodies inhibit the acquisition of Borrelia burgdorferi by Ixodes ticks. Infect. Immun. 65:3146-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Silva, A. M., S. R. Telford III, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fikrig, E., S. W. Barthold, and R. A. Flavell. 1993. OspA vaccination of mice with established Borrelia burgdorferi infection alters disease but not infection. Infect. Immun. 61:2553-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fikrig, E., S. R. Telford III, S. W. Barthold, F. S. Kantor, A. Spielman, and R. A. Flavell. 1992. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proc. Natl. Acad. Sci. USA 89:5418-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilmore, R. D., Jr., M. L. Mbow, and B. Stevenson. 2001. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 13.Kochi, S. K., R. C. Johnson, and A. P. Dalmasso. 1991. Complement-mediated killing of the Lyme disease spirochete Borrelia burgdorferi. Role of antibody in formation of an effective membrane attack complex. J. Immunol. 146:3964-3970. [PubMed] [Google Scholar]
- 14.Kraiczy, P., C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur. J. Immunol. 31:1674-1684. [DOI] [PubMed] [Google Scholar]
- 15.Kraiczy, P., C. Skerka, M. Kirschfink, P. F. Zipfel, and V. Brade. 2001. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int. Immunopharmacol. 1:393-401. [DOI] [PubMed] [Google Scholar]
- 16.Kurtenbach, K., S. De Michelis, S. Etti, S. M. Schafer, H. S. Sewell, V. Brade, and P. Kraiczy. 2002. Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol. 10:74-79. [DOI] [PubMed] [Google Scholar]
- 17.Kurtenbach, K., H. S. Sewell, N. H. Ogden, S. E. Randolph, and P. A. Nuttall. 1998. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect. Immun. 66:1248-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margos, G., S. Navarette, G. Butcher, A. Davies, C. Willers, R. E. Sinden, and P. J. Lachmann. 2001. Interaction between host complement and mosquito-midgut-stage Plasmodium berghei. Infect. Immun. 69:5064-5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nowling, J. M., and M. T. Philipp. 1999. Killing of Borrelia burgdorferi by antibody elicited by OspA vaccine is inefficient in the absence of complement. Infect. Immun. 67:443-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal, U., A. M. de Silva, R. R. Montgomery, D. Fish, J. Anguita, J. F. Anderson, Y. Lobet, and E. Fikrig. 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Investig. 106:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patarakul, K., M. F. Cole, and C. A. Hughes. 1999. Complement resistance in Borrelia burgdorferi strain 297: outer membrane proteins prevent MAC formation at lysis susceptible sites. Microb. Pathog. 27:25-41. [DOI] [PubMed] [Google Scholar]
- 22.Philipp, M. T. 1998. Studies on OspA: a source of new paradigms in Lyme disease research. Trends Microbiol. 6:44-47. [DOI] [PubMed] [Google Scholar]
- 23.Piesman, J. 1993. Standard system for infecting ticks (Acari: Ixodidae) with the Lyme disease spirochete, Borrelia burgdorferi. J. Med. Entomol. 30:199-203. [DOI] [PubMed] [Google Scholar]
- 24.Rathinavelu, S., and A. M. de Silva. 2001. Purification and characterization of Borrelia burgdorferi from feeding nymphal ticks (Ixodes scapularis). Infect. Immun. 69:3536-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribeiro, J. M. 1987. Ixodes dammini: salivary anti-complement activity. Exp. Parasitol. 64:347-353. [DOI] [PubMed] [Google Scholar]
- 26.Richards, A. G., and P. A. Richards. 1977. The peritrophic membranes of insects. Annu. Rev. Entomol. 22:219-240. [DOI] [PubMed] [Google Scholar]
- 27.Schwan, T. G. 1996. Ticks and Borrelia: model systems for investigating pathogen-arthropod interactions. Infect. Agents Dis. 5:167-181. [PubMed] [Google Scholar]
- 28.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sole, M., C. Bantar, K. Indest, Y. Gu, R. Ramamoorthy, R. Coughlin, and M. T. Philipp. 1998. Borrelia burgdorferi escape mutants that survive in the presence of antiserum to the OspA vaccine are killed when complement is also present. Infect. Immun. 66:2540-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevenson, B., N. El-Hage, M. A. Hines, J. C. Miller, and K. Babb. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarnowski, B. I., and L. B. Coons. 1989. Ultrastructure of the midgut and blood meal digestion in the adult tick Dermacentor variabilis. Exp. Appl. Acarol. 6:263-289. [DOI] [PubMed] [Google Scholar]
- 32.Tsuboi, T., Y. M. Cao, M. Torii, Y. Hitsumoto, and H. Kanbara. 1995. Murine complement reduces infectivity of Plasmodium yoelii to mosquitoes. Infect. Immun. 63:3702-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valenzuela, J. G., R. Charlab, T. N. Mather, and J. M. Ribeiro. 2000. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J. Biol. Chem. 275:18717-18723. [DOI] [PubMed] [Google Scholar]
- 34.van Dam, A. P., A. Oei, R. Jaspars, C. Fijen, B. Wilske, L. Spanjaard, and J. Dankert. 1997. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect. Immun. 65:1228-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wessels, M. R., P. Butko, M. Ma, H. B. Warren, A. L. Lage, and M. C. Carroll. 1995. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA 92:11490-11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wikel, S. K. 1999. Tick modulation of host immunity: an important factor in pathogen transmission. Int. J. Parasitol. 29:851-859. [DOI] [PubMed] [Google Scholar]