Abstract
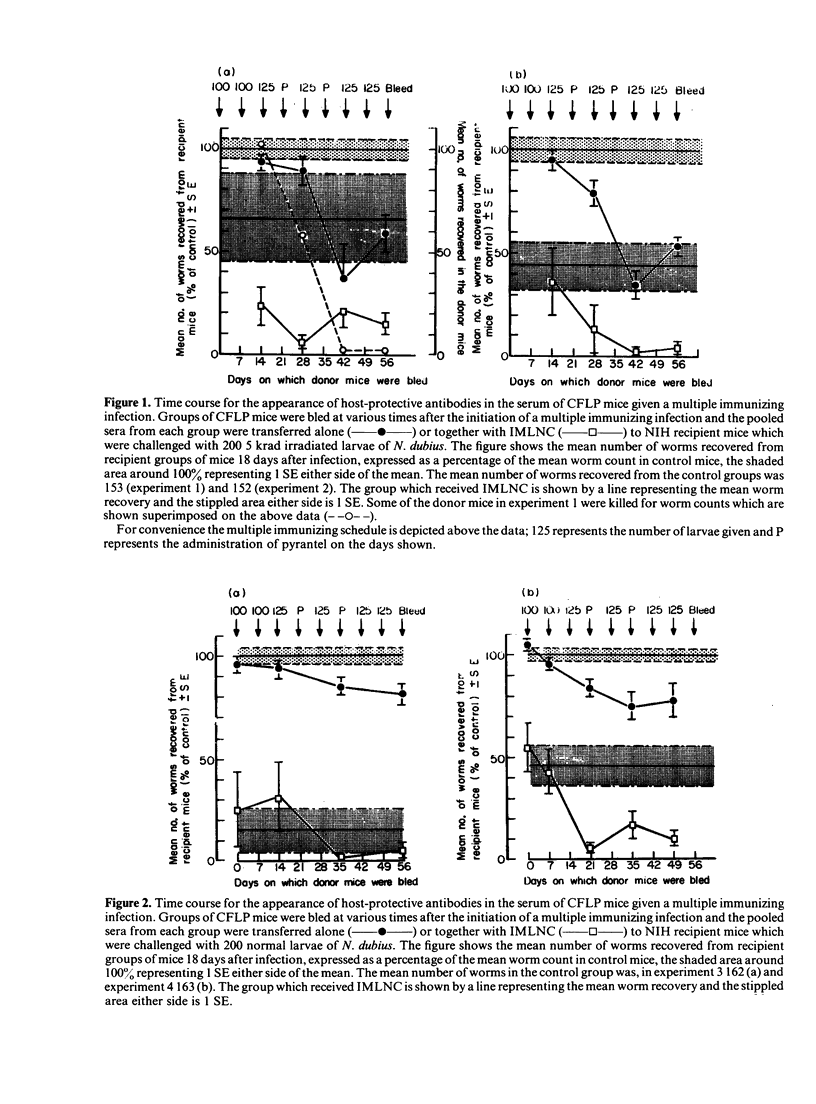
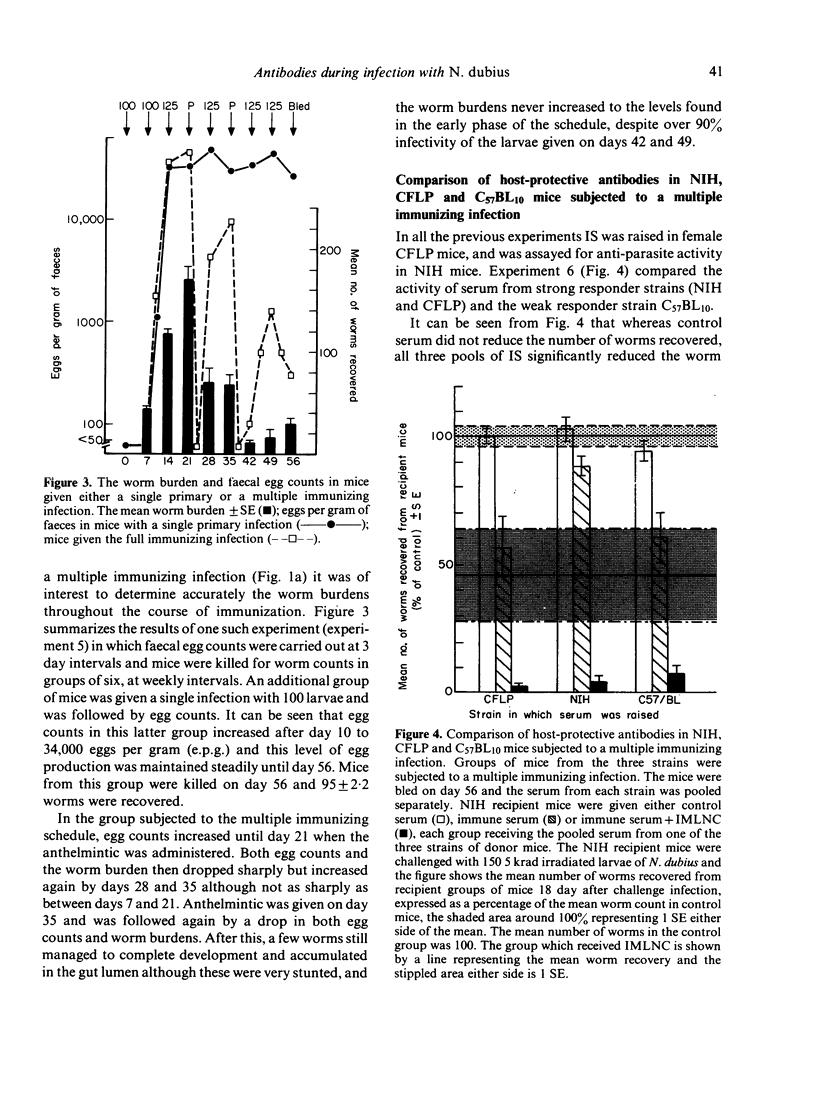
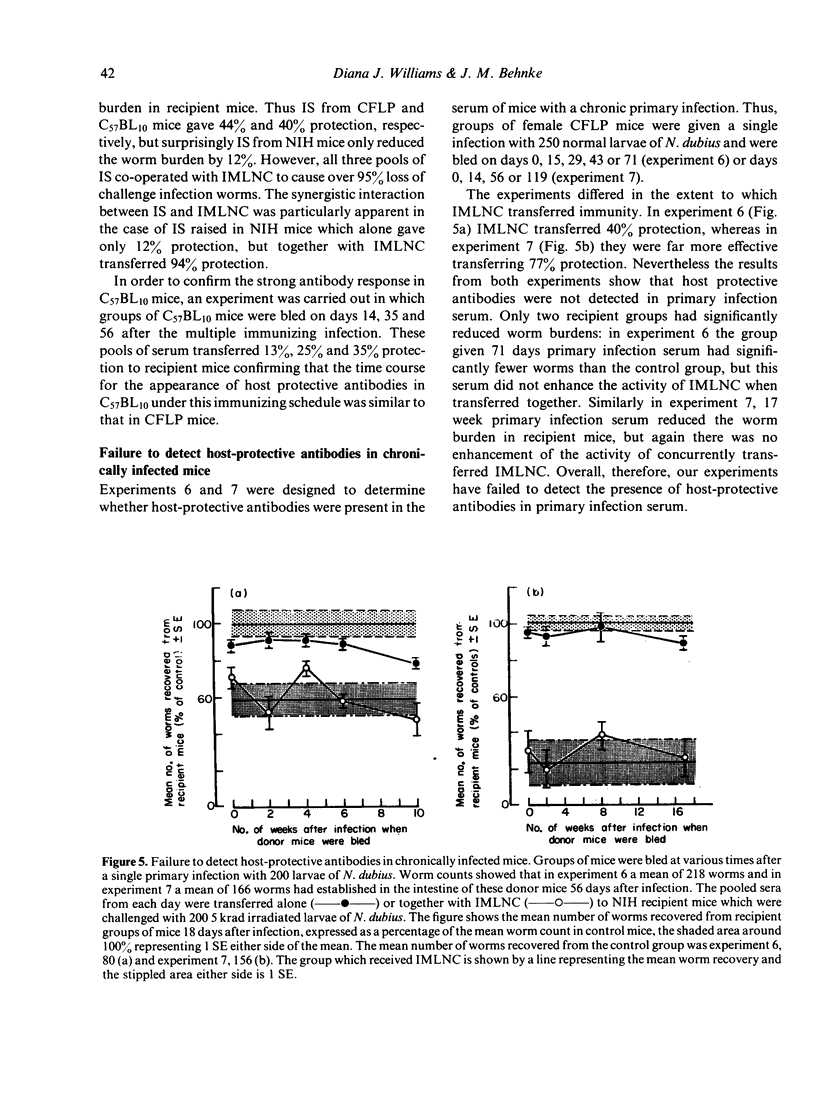
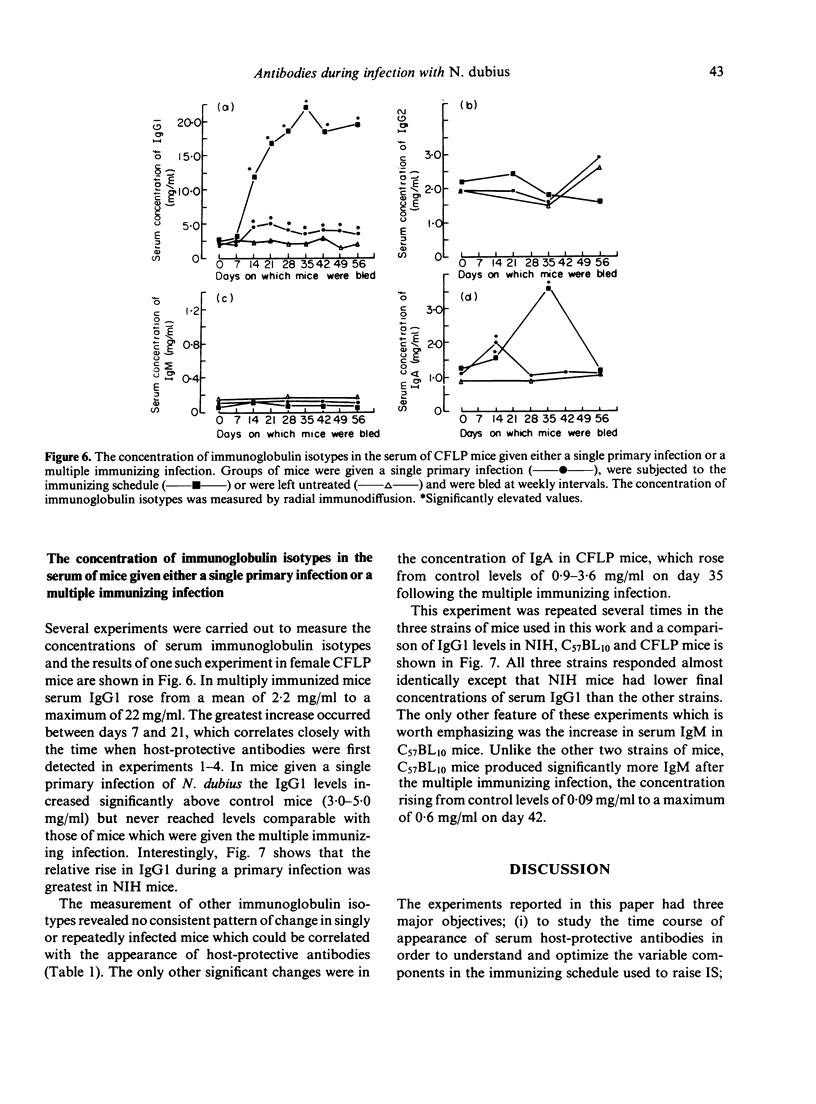
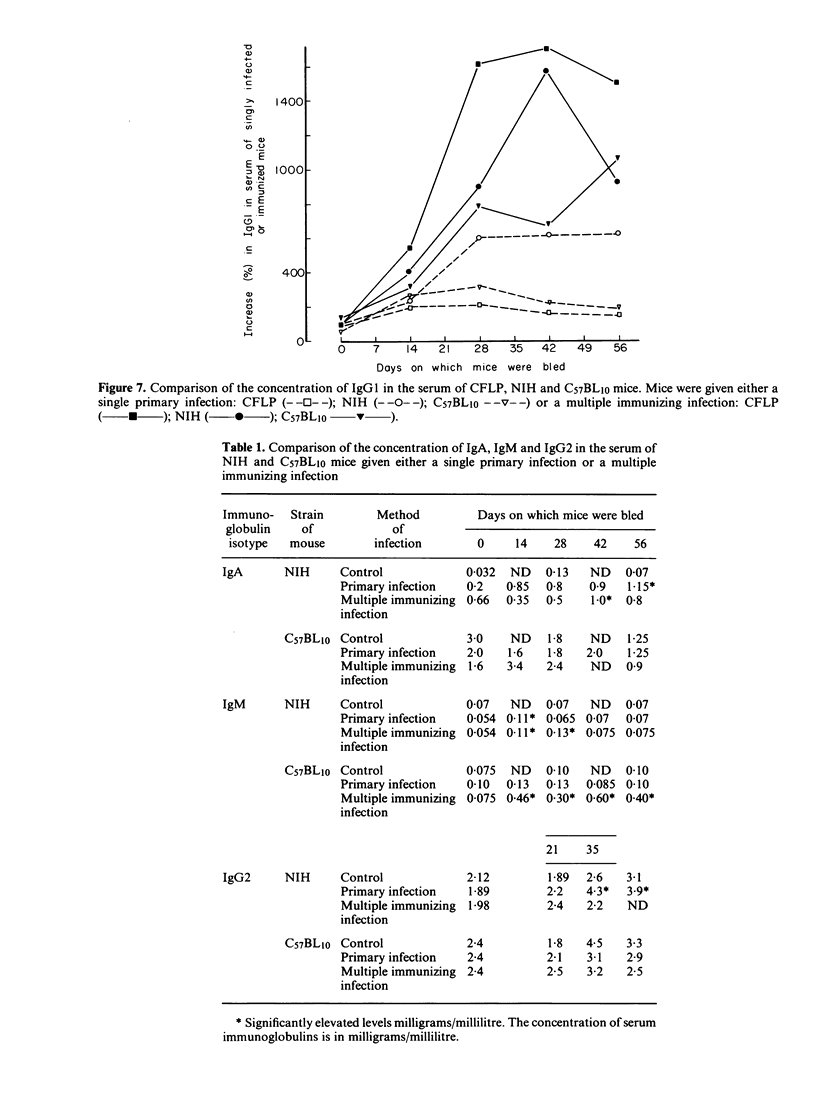
The nematode parasite Nematospiroides dubius survives to give a chronic primary infection in mice. However, mice subjected to weekly infections of 125 larvae, interspersed by treatment with an anthelmintic to prevent the accumulation of lethal numbers of adult worms in the intestine, develop host-protective antibodies in their serum. The protective effect of these antibodies was demonstrated by passive transfer to naive recipients or to mice already adoptively immunized with immune mesenteric lymph node cells (IMLNC). Sera were first shown to exhibit protective activity during the third and fourth weeks of the multiple immunizing infection, reaching a peak level by week six beyond which there was no further increase in protective activity. This increase was correlated with a ten-fold, concurrent rise in serum IgG1 levels. None of the other immunoglobulin isotypes underwent comparable changes in concentration nor could they be correlated with the pattern of appearance of host-protective antibodies in the sera of donor mice. This suggested that host protective antibodies were of the IgG1 class. CFLP and C57BL10 mice (the latter is a weak responder strain) both had high levels of host-protective antibodies in their serum. However when the sera from NIH mice (a strong responder strain) were compared, they exhibited far less protective activity on passive transfer to recipient mice, although when given together with IMLNC, serum from multiply-immunized NIH mice enhanced the protective effect of IMLNC synergistically. When primary infection serum was assayed in this passive/adoptive transfer model, no host-protective antibodies could be demonstrated, even with pools of primary infection serum taken 10 and 17 weeks after infection. These results are discussed with respect to the possible mechanisms by which N. dubius evades the host immune system to give rise to long-lasting primary infections in mice.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behnke J. M., Parish H. A. Expulsion of Nematospiroides dubius from the intestine of mice treated with immune serum. Parasite Immunol. 1979 Spring;1(1):13–26. doi: 10.1111/j.1365-3024.1979.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Behnke J. M., Parish H. A., Hagan P. The effect of gamma irradiation on Nematospiroides dubius. Factors affecting the survival of worms in a primary infection in mice. J Helminthol. 1980 Sep;54(3):173–182. doi: 10.1017/s0022149x00006556. [DOI] [PubMed] [Google Scholar]
- Behnke J. M., Parish H. A. Nematospiroides dubius: arrested development of larvae in immune mice. Exp Parasitol. 1979 Feb;47(1):116–127. doi: 10.1016/0014-4894(79)90013-4. [DOI] [PubMed] [Google Scholar]
- Behnke J. M., Parish H. A. Transfer of immunity to Nematospiroides dubius: co-operation between lymphoid cells and antibodies in mediating worm expulsion. Parasite Immunol. 1981 Autumn;3(3):249–259. doi: 10.1111/j.1365-3024.1981.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Behnke J. M., Wakelin D. Nematospiroides dubius: stimulation of acquired immunity in inbred strains of mice. J Helminthol. 1977 Sep;51(3):167–176. doi: 10.1017/s0022149x0000746x. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E., David J. R., Franks D., Mahmoud A. A., David P. H., Sturrock R. F., Houba V. Antibody-dependent eosinophil-mediated damage to 51Cr-labeled schistosomula of Schistosoma mansoni: damage by purieid eosinophils. J Exp Med. 1977 Jan 1;145(1):136–150. doi: 10.1084/jem.145.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman C. B., Knopf P. M., Anders R. F., Mitchell G. F. IgG1 hypergammaglobulinaemia in chronic parasitic infections in mice: evidence that the response reflects chronicity of antigen exposure. Aust J Exp Biol Med Sci. 1979 Aug;57(4):389–400. doi: 10.1038/icb.1979.39. [DOI] [PubMed] [Google Scholar]
- Prowse S. J., Mitchell G. F., EY P. L., Jenkin C. R. The development of resistance in different inbred strains of mice to infection with Nematospiroides dubius. Parasite Immunol. 1979 Winter;1(4):277–288. doi: 10.1111/j.1365-3024.1979.tb00713.x. [DOI] [PubMed] [Google Scholar]
- Wakelin D., Donachie A. M. Genetic control of immunity to Trichinella spiralis. Donor bone marrow cells determine responses to infection in mouse radiation chimaeras. Immunology. 1981 Aug;43(4):787–792. [PMC free article] [PubMed] [Google Scholar]
- Wakelin D., Donachie A. M. Genetic control of immunity to parasites: adoptive transfer of immunity between inbred strains of mice characterized by rapid and slow immune expulsion of Trichinella spiralis. Parasite Immunol. 1980 Winter;2(4):249–260. doi: 10.1111/j.1365-3024.1980.tb00057.x. [DOI] [PubMed] [Google Scholar]


