Abstract
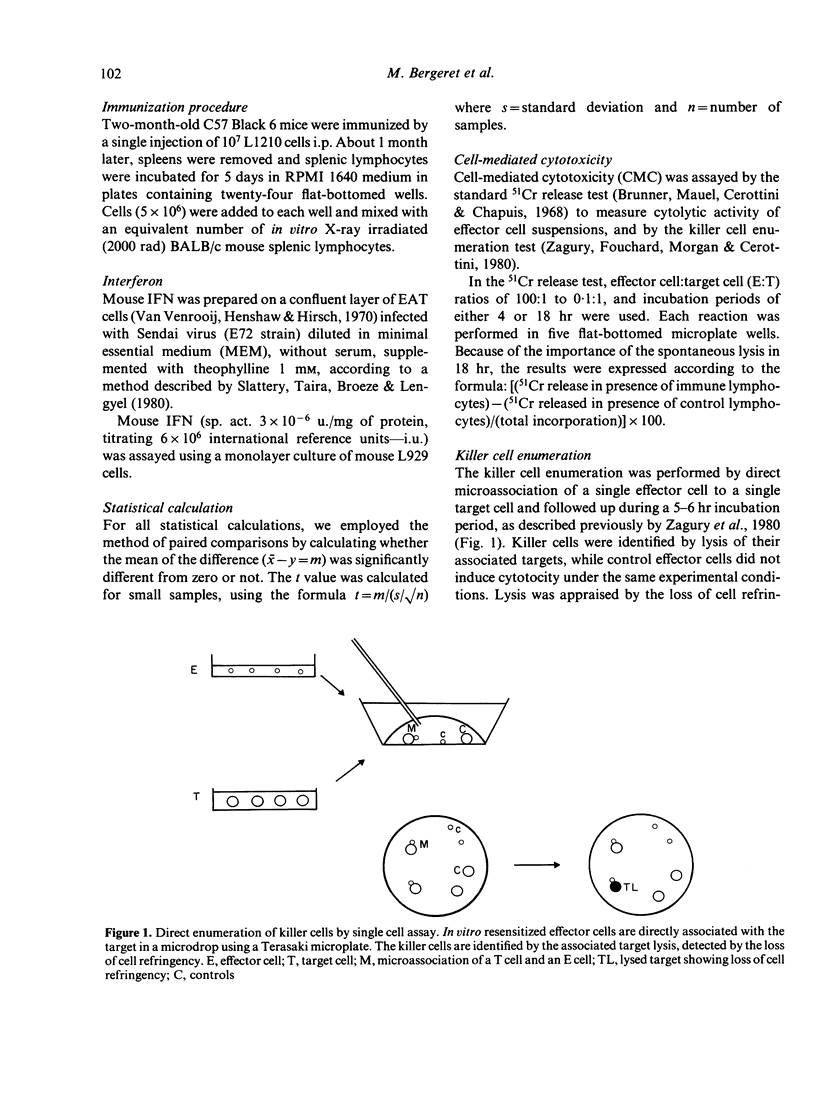
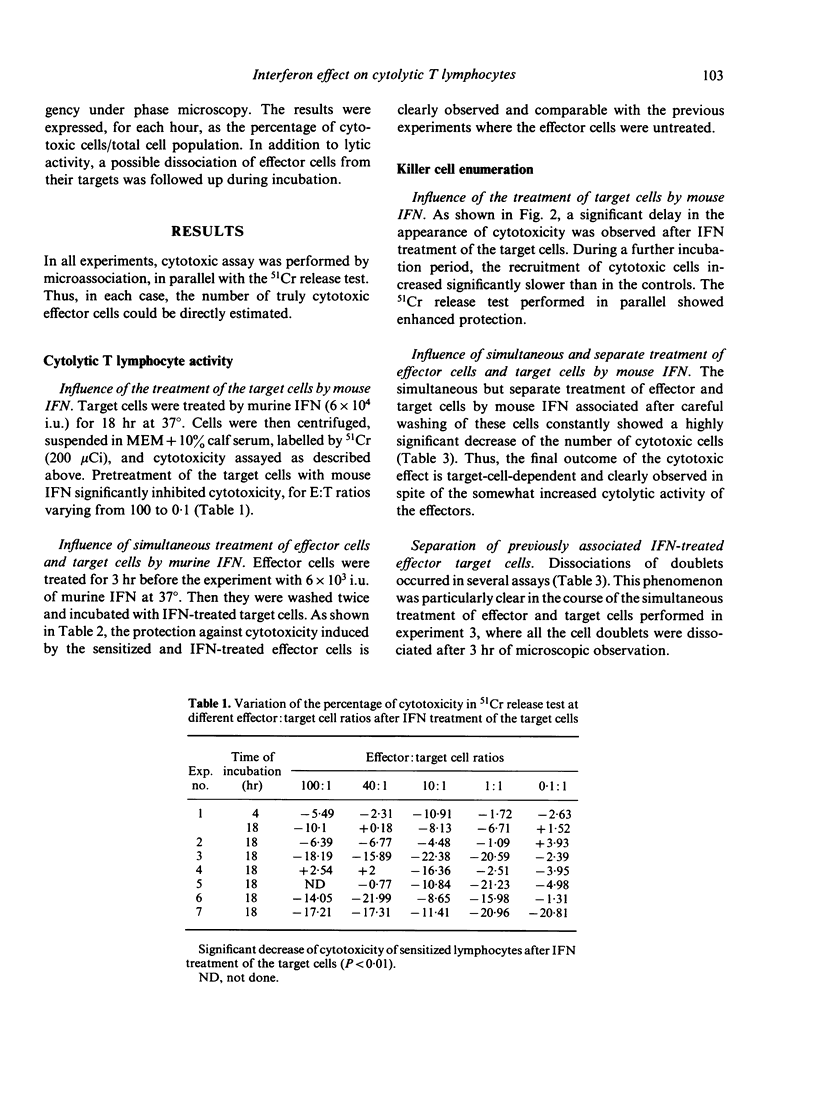
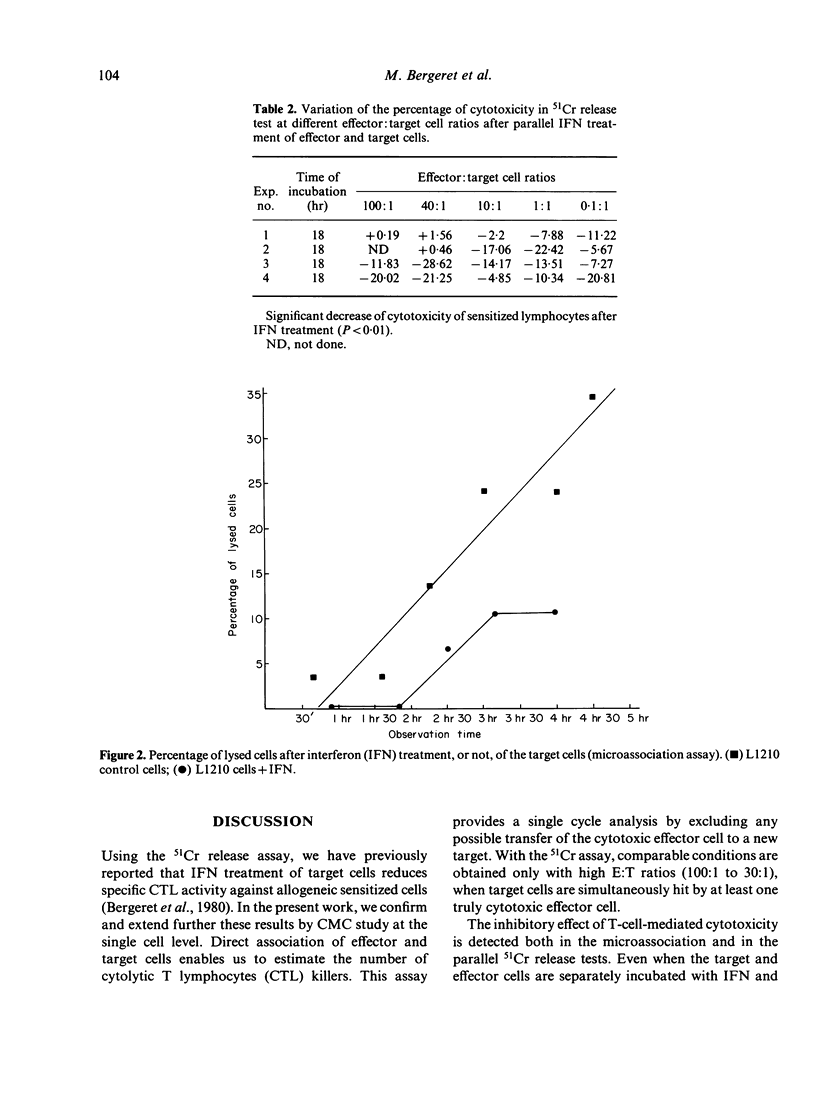
In this study we analyse cell-mediated cytotoxicity by the use of 51chromium release and the killer cell enumeration assay. The latter enabled us to estimate cell-mediated cytotoxicity in a single cycle, which confirms the protective effect of interferon (IFN) after target cell treatment. This anti-cytolytic resistance is detected best by microassociation when effector and target cells are separately treated with IFN and associated thereafter. We suggest that, in inflammatory areas, enhanced cytotoxic activity of the IFN-treated effector cells is only operative before the establishment of protection in the targets, which is somewhat slower and appears in about 18 hr. Resistance of the targets could be at least partly attributed to the incapacity of effector cells to bind to them.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergeret M., Grégoire A., Chany C. Protective effect of interferon on target cells exposed to cellular cytotoxicity. Immunology. 1980 Aug;40(4):637–643. [PMC free article] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Cerutti I., Chany C., Schlumberger J. F. Isoprinosine increases the antitumor action of interferon. Int J Immunopharmacol. 1979;1(1):59–63. doi: 10.1016/0192-0561(79)90031-6. [DOI] [PubMed] [Google Scholar]
- Heron I., Berg K., Cantell K. Regulatory effect of interferon on T cells in vitro. J Immunol. 1976 Oct;117(4):1370–1373. [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the specific cytotoxicity of sensitized lymphocytes. Proc Natl Acad Sci U S A. 1972 Mar;69(3):721–725. doi: 10.1073/pnas.69.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M., White W. J., Potter M. R. Modulation of target cell susceptibility to human natural killer cells by interferon. Int J Cancer. 1980 May 15;25(5):565–572. doi: 10.1002/ijc.2910250504. [DOI] [PubMed] [Google Scholar]
- PAUCKER K., CANTELL K., HENLE W. Quantitative studies on viral interference in suspended L cells. III. Effect of interfering viruses and interferon on the growth rate of cells. Virology. 1962 Jun;17:324–334. doi: 10.1016/0042-6822(62)90123-x. [DOI] [PubMed] [Google Scholar]
- Slattery E., Taira H., Broeze R., Lengyel P. Mouse interferons: production by Ehrlich ascites tumour cells infected with Newcastle disease virus and its enhancement by theophylline. J Gen Virol. 1980 Jul;49(1):91–96. doi: 10.1099/0022-1317-49-1-91. [DOI] [PubMed] [Google Scholar]
- Svet-Moldavsky G. J., Chernyakhovskaya I. J. Interferon and the interaction of allogeneic normal and immune lymphocytes with L-cells. Nature. 1967 Sep 16;215(5107):1299–1300. doi: 10.1038/2151299a0. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978 May 1;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ventrooij W. J., Henshaw E. C., Hirsch C. A. Nutritional effects on the polyribosome distribution and rate of protein synthesis in Ehrlich ascites tumor cells in culture. J Biol Chem. 1970 Nov 25;245(22):5947–5953. [PubMed] [Google Scholar]


