Abstract
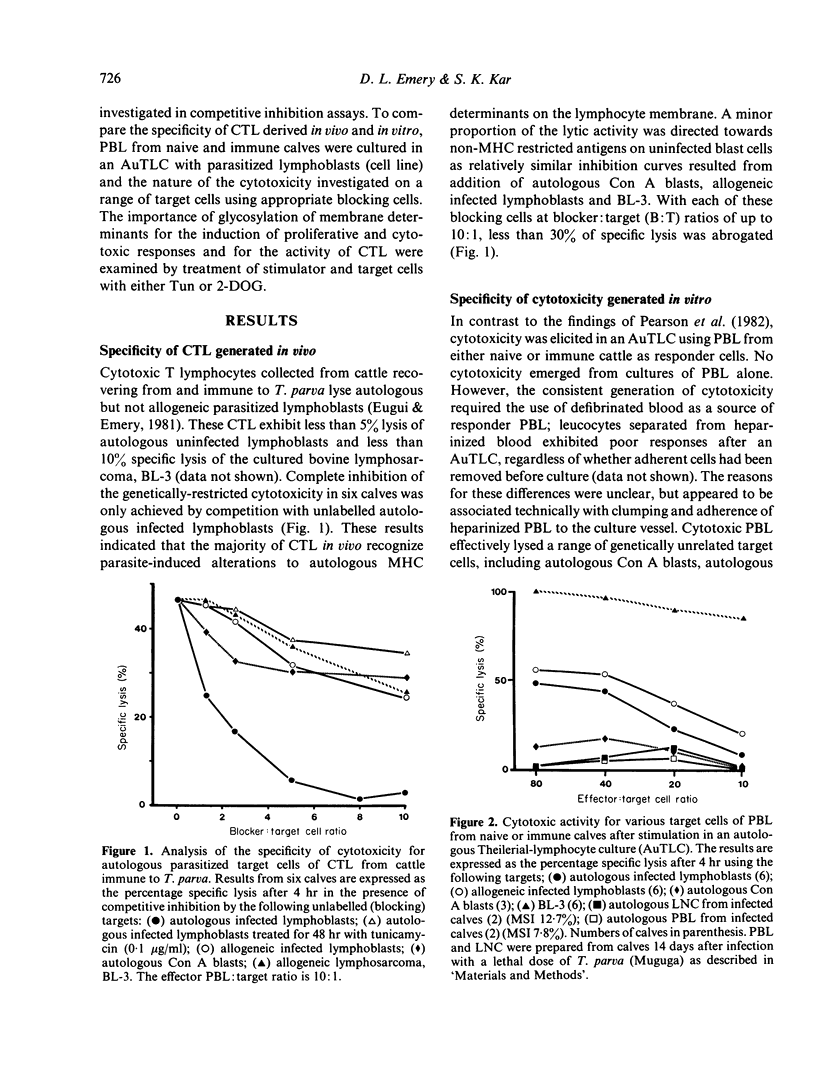
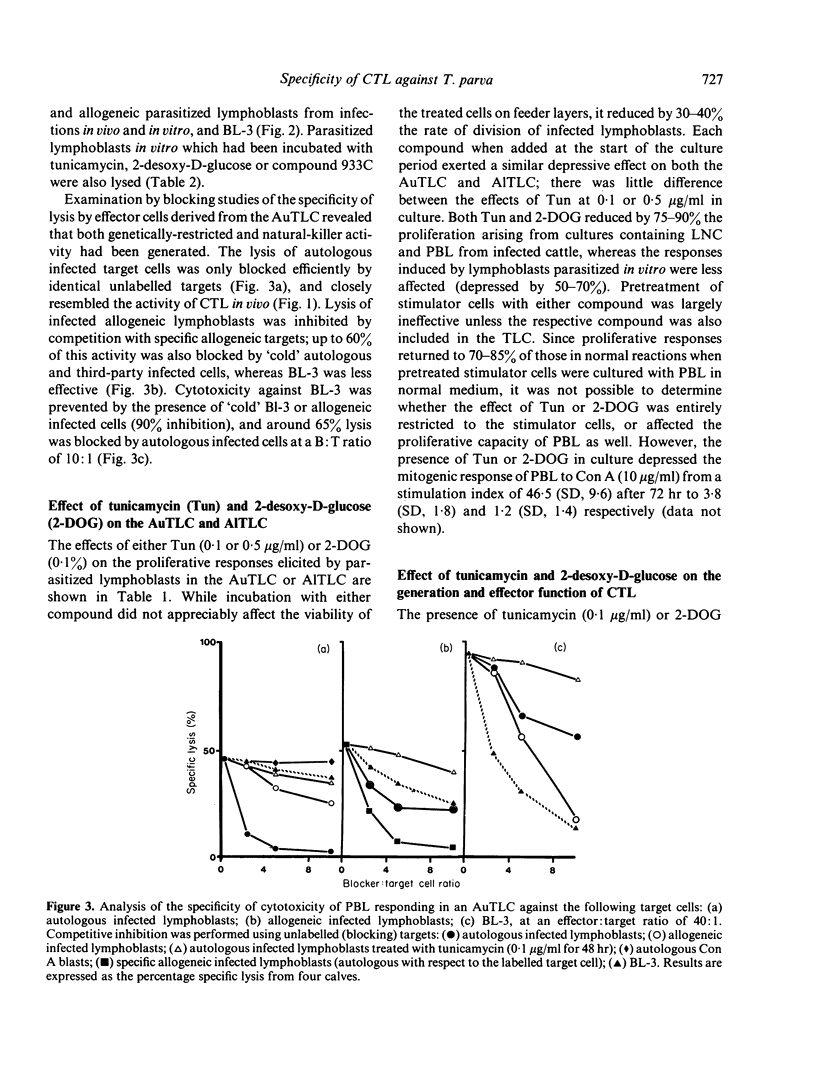
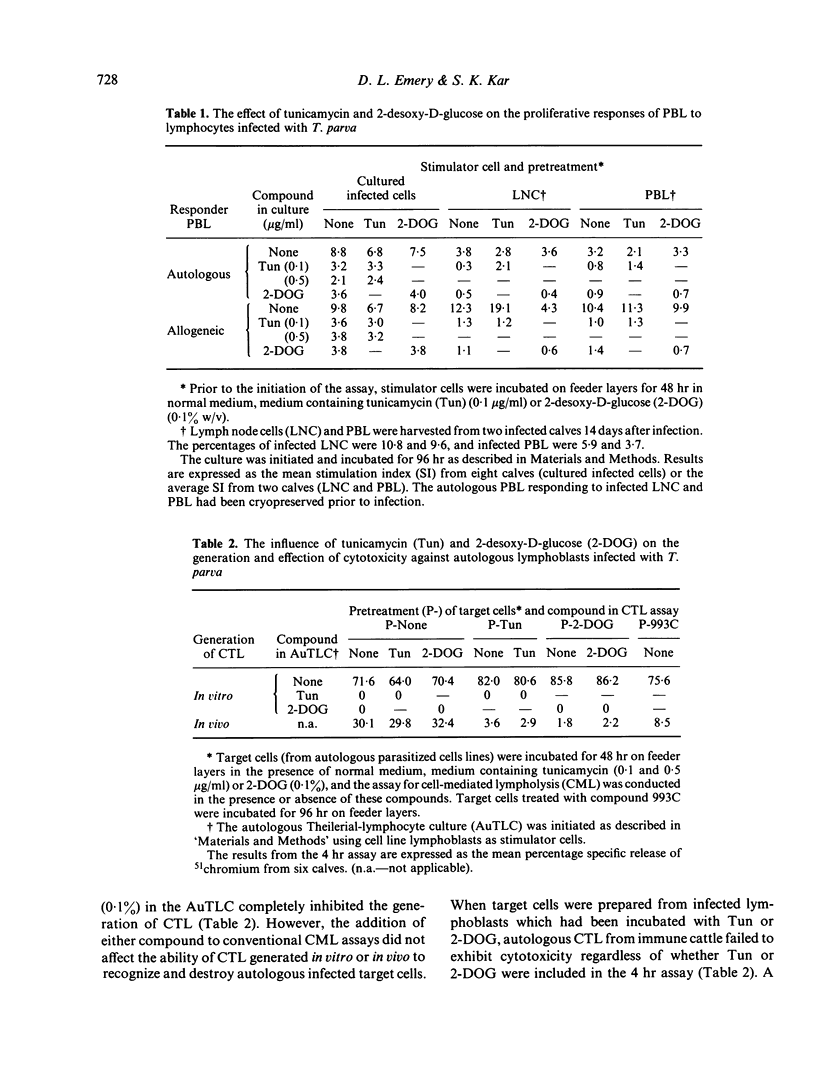
Examination of the specificity of cytotoxicity generated in vitro and in vivo against infected bovine lymphoblasts revealed that cytotoxic T lymphocytes (CTL) obtained from cattle immune to Theileria parva recognized parasite-induced alterations associated with major histocompatibility complex (MHC) antigens on the membrane of infected autologous cells. By comparison, cytotoxicity generated in vitro in an autologous Theilerial-lymphocyte culture (AuTLC) contained both CTL and activity akin to that of natural-killer (NK) cells. The addition to the AuTLC of 2 inhibitors of glycosylation, tunicamycin (Tun) and 2-desoxy-D-glucose (2-DOG) abolished both the proliferative response and the generation of cytotoxicity. While the addition of Tun or 2-DOG in conventional cell-mediated lympholysis (CML) assays did not modify the effector function of cytotoxic cells, pretreatment of target cells with either compound prevented lysis by CTL, but not by NK cells. Although parasite-induced antigens have not been purified from infected bovine lymphoblasts, the present study indicated that these are likely to be glycoprotein or carbohydrate in character, and that their recognition on autologous cells is a consistent feature of CTL from immune cattle.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black P. L., Vitetta E. S., Forman J., Kang C. Y., May R. D., Uhr J. W. Role of glycosylation in the H-2-restricted cytolysis of virus-infected cells. Eur J Immunol. 1981 Jan;11(1):48–55. doi: 10.1002/eji.1830110111. [DOI] [PubMed] [Google Scholar]
- Bolhuis R. L., Schellekens H. Induction of natural killer cell activity and allocytotoxicity in human peripheral blood lymphocytes after mixed lymphocyte culture. Scand J Immunol. 1981;13(5):401–412. doi: 10.1111/j.1365-3083.1981.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Braciale T. J. Immunologic recognition of influenza virus-infected cells. II. Expression of influenza A matrix protein on the infected cell surface and its role in recognition by cross-reactive cytotoxic T cells. J Exp Med. 1977 Sep 1;146(3):673–689. doi: 10.1084/jem.146.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. G., Stagg D. A., Purnell R. E., Kanhai G. K., Payne R. C. Letter: Infection and transformation of bovine lymphoid cells in vitro by infective particles of Theileria parva. Nature. 1973 Sep 14;245(5420):101–103. doi: 10.1038/245101a0. [DOI] [PubMed] [Google Scholar]
- Callewaert D. M., Lightbody J. J., Kaplan J., Jaroszewski J., Peterson W. D., Jr, Rosenberg J. C. Cytotoxicity of human peripheral lymphocytes in cell-mediated lympholysis; antibody-dependent cell-mediated lympholysis and natural cytotoxicity assays after mixed lymphocyte culture. J Immunol. 1978 Jul;121(1):81–85. [PubMed] [Google Scholar]
- Cantor H., Jandinski J. The relationship of cell division to the generation of cytotoxic activity in mixed lymphocyte culture. J Exp Med. 1974 Dec 1;140(6):1712–1716. doi: 10.1084/jem.140.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R. J., Steiner S. M., Benyesh-Melnick M. Effects of 2-deoxy-D-glucose on herpes simplex virus replication. Virology. 1973 Apr;52(2):447–455. doi: 10.1016/0042-6822(73)90340-1. [DOI] [PubMed] [Google Scholar]
- Creemers P. Lack of reactivity of sera from Theileria parva-infected and recovered cattle against cell membrane antigens of Theileria parva transformed cell lines. Vet Immunol Immunopathol. 1982 Jul;3(4):427–438. doi: 10.1016/0165-2427(82)90025-3. [DOI] [PubMed] [Google Scholar]
- Emery D. L., Eugui E. M., Nelson R. T., Tenywa T. Cell-mediated immune responses to Theileria parva (East Coast fever) during immunization and lethal infections in cattle. Immunology. 1981 Jun;43(2):323–336. [PMC free article] [PubMed] [Google Scholar]
- Emery D. L., Morrison W. I. Generation of autologous mixed leucocyte reactions during the course of infection with Theileria parva (East Coast Fever) in cattle. Immunology. 1980 Jun;40(2):229–237. [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A., Martin W. J., Verbonitz M. W. Hemagglutinin-specific cytotoxic T-cell response during influenza infection. J Exp Med. 1977 Sep 1;146(3):893–898. doi: 10.1084/jem.146.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugui E. M., Emery D. L. Genetically restricted cell-mediated cytotoxicity in cattle immune to Theileria parva. Nature. 1981 Mar 19;290(5803):251–254. doi: 10.1038/290251a0. [DOI] [PubMed] [Google Scholar]
- HULLIGER L., WILDE K. H., BROWN C. G., TURNER L. MODE OF MULTIPLICATION OF THEILERIA IN CULTURES OF BOVINE LYMPHOCYTIC CELLS. Nature. 1964 Aug 15;203:728–730. doi: 10.1038/203728a0. [DOI] [PubMed] [Google Scholar]
- Hale A. H. H-2 antigens incorporated into phospholipid vesicles elicit specific allogeneic cytotoxic T lymphocytes. Cell Immunol. 1980 Oct;55(2):328–341. doi: 10.1016/0008-8749(80)90165-3. [DOI] [PubMed] [Google Scholar]
- Jackson D. C., Ada G. L., Hapel A. J., Dunlop M. B. Changes in the surface of virus-infected cells recognized by cytotoxic T cells. II. A requirement for glycoprotein synthesis in virus-infected target cells. Scand J Immunol. 1976;5(9):1021–1029. doi: 10.1111/j.1365-3083.1976.tb03054.x. [DOI] [PubMed] [Google Scholar]
- Lawman M. J., Courtney R. J., Eberle R., Schaffer P. A., O'Hara M. K., Rouse B. T. Cell-mediated immunity to herpes simplex virus: specificity of cytotoxic T cells. Infect Immun. 1980 Nov;30(2):451–461. doi: 10.1128/iai.30.2.451-461.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee M., Hale A. H., Panetti M. Elicitation of primary anti-Sendai virus cytotoxic T lymphocytes with purified viral glycoproteins. Eur J Immunol. 1980 Dec;10(12):923–928. doi: 10.1002/eji.1830101207. [DOI] [PubMed] [Google Scholar]
- McHardy N. In vitro studies on the action of menoctone and other compounds on Theileria parva and T. annulata. Ann Trop Med Parasitol. 1978 Dec;72(6):501–511. doi: 10.1080/00034983.1978.11719354. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F., Cullen P., Askonas B., Webster R. G. The human cytotoxic T cell response to influenza A vaccination. Clin Exp Immunol. 1981 Feb;43(2):276–284. [PMC free article] [PubMed] [Google Scholar]
- Morrison W. I., Buscher G., Murray M., Emery D. L., Masake R. A., Cook R. H., Wells P. W. Theileria parva: kinetics of infection in the lymphoid system of cattle. Exp Parasitol. 1981 Oct;52(2):248–260. doi: 10.1016/0014-4894(81)90080-1. [DOI] [PubMed] [Google Scholar]
- Payne L. N., Powell P. C., Rennie M. C., Ross L. J. Studies on the mechanism of vaccinal immunity to Marek's disease. Comp Immunol Microbiol Infect Dis. 1978;1(1-2):31–36. doi: 10.1016/0147-9571(78)90007-3. [DOI] [PubMed] [Google Scholar]
- Pearson T. W., Hewett R. S., Roelants G. E., Stagg D. A., Dolan T. T. Studies on the induction and specificity of cytotoxicity to Theileria-transformed cell lines. J Immunol. 1982 Jun;128(6):2509–2513. [PubMed] [Google Scholar]
- Pearson T. W., Lundin L. B., Dolan T. T., Stagg D. A. Cell-mediated immunity to Theileria-transformed cell lines. Nature. 1979 Oct 25;281(5733):678–680. doi: 10.1038/281678a0. [DOI] [PubMed] [Google Scholar]
- Sugamura K., Hinuma Y. In vitro induction of cytotoxic T lymphocytes specific for Epstein-Barr virus-transformed cells: kinetics of autologous restimulation. J Immunol. 1980 Mar;124(3):1045–1049. [PubMed] [Google Scholar]
- Viallat J., Svedmyr E., Steinitz M., Klein G. Stimulation of peripheral human lymphocytes by autologous EBV genome-carrying lymphoblastoid cell lines. Cell Immunol. 1978 Jun;38(1):68–75. doi: 10.1016/0008-8749(78)90032-1. [DOI] [PubMed] [Google Scholar]


